 Case Report
Case Report
Infliximab Redosing During Massive GI Bleeding Resulting from Severe Crohn’s Disease
Niharika Mallepally, Vijay Prabhakar, Sarah Sheibani and Jacques Van Dam*
Department of Gastroenterology, Keck School of Medicine of USC, USA
Jacques Van Dam, Department of Gastroenterology, Keck School of Medicine of USC, USA.
Received Date: October 27, 2022; Published Date: November 18, 2022
Introduction
In moderate-to-severe Crohn’s disease (CD), early introduction of biologics is indicated. However, a standard frequency induction regimen may be insufficient for disease activity control in patients with significant ongoing hematochezia. We present a previously healthy 21-year-old man who was found to have severe-fulminant CD with involvement of the esophagus, stomach, small intestine, and colon. Since his disease remained refractory despite an accelerated high-dose infliximab initiation regimen, an infliximab level was checked and returned undetectable. In patients with Crohn’s disease who present with large volume gastrointestinal hemorrhage, it is imperative to proactively assess infliximab levels and aggressively re-dose if needed.
Case Report
Severe gastrointestinal hemorrhage is a rare complication of Crohn’s disease (incidence 0.6-4%) [1]. Early introduction of biologics is indicated in moderate-to-severe CD; however, literature rarely reports on the utility of an accelerated infliximab regimen (frequency exceeding recommendation in product monograph) [2,3]. Fecal loss of infliximab in severe disease has been reported [4], and large volume enteral bleeding likely also impacts the serum infliximab level. Here, we present the case of a critically ill patient with severe-fulminant CD and ongoing significant gastrointestinal hemorrhage. He was found to have an undetectable infliximab level despite an accelerated, high-dose infliximab induction regimen.
Case Report
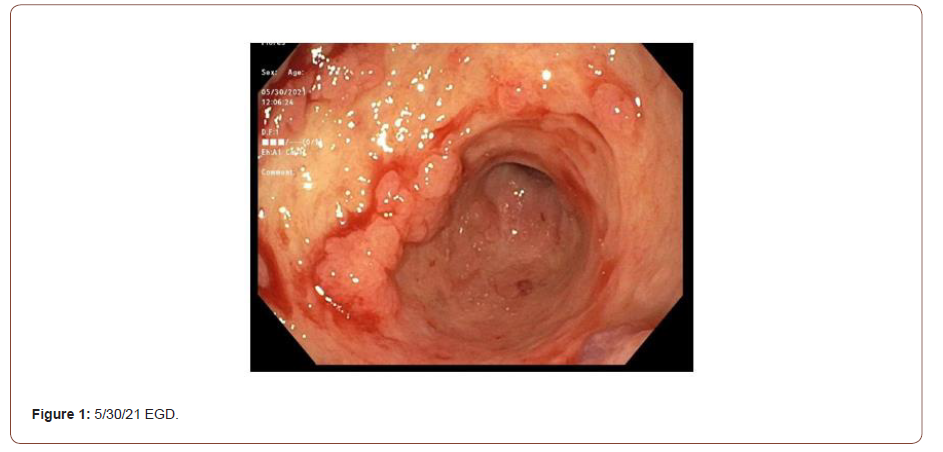
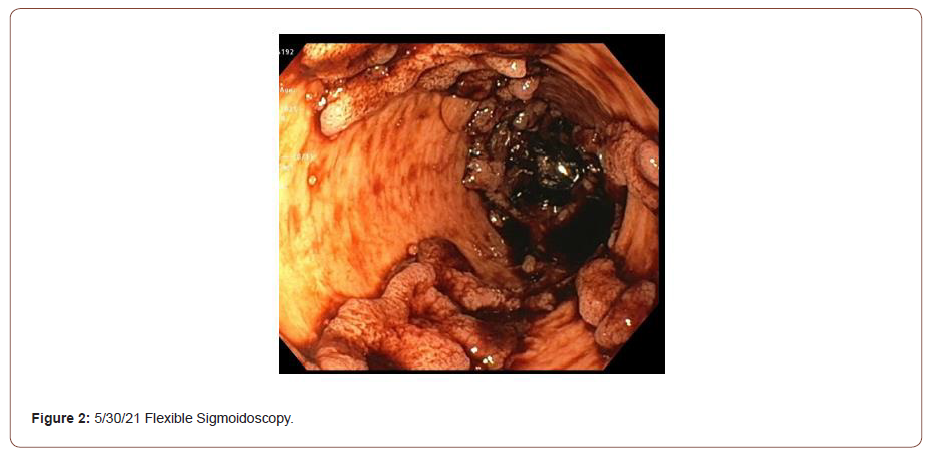
The patient arrived from an outside hospital on a course of mesalamine and prednisone with worsening hematochezia and hemodynamic instability. An EGD showed severe Los Angeles Grade-D esophagitis, cratered gastric ulcers, and contiguous serpiginous duodenal ulcers with pseudo polyps and diffuse oozing (Figure 1). Pathology revealed Crohn’s disease, as well as H. pylori infection. A flexible sigmoidoscopy showed erythema, friability, and pseudopolyps (Figure 2). Also noted were confluent, deep, and serpentine ulcerations in a circumferential pattern from the anus to the proximal sigmoid colon. Pathology showed marked crypt architectural changes and cytomegalovirus (CMV). Given diffuse mucosal bleeding, the patient underwent interventional radiological (IR) embolization of a branch of the superior pancreaticoduodenal artery (PDA). For CD, high dose infliximab (10 mg/kg) and IV steroids were initiated. Ganciclovir was started for CMV. Two days later, continued hematochezia led to the initiation of vasopressors and an IR embolization of an inferior branch of the PDA. For the next week, the patient had melena, hematochezia, and tachycardia with a hemoglobin nadir of 5.9 g/dL. A CT angiography (CTA) showed extravasation near the fourth segment of the duodenum; however, the vessel was not embolized due to poor localization.
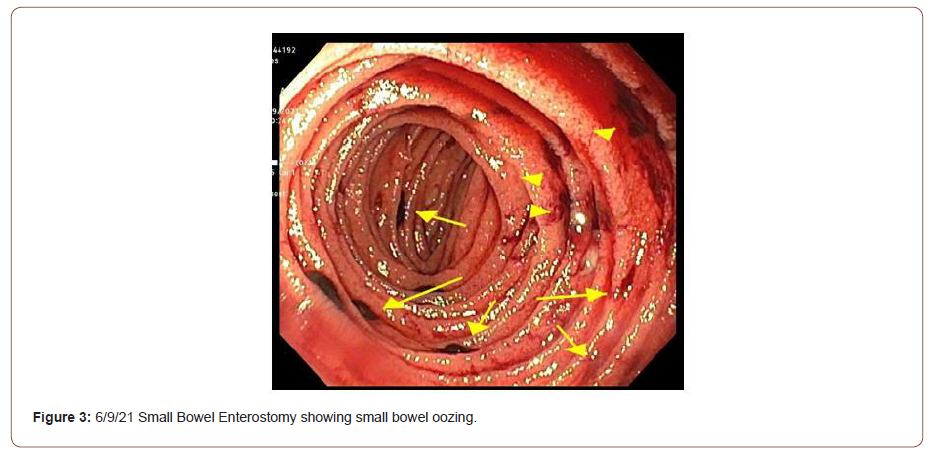
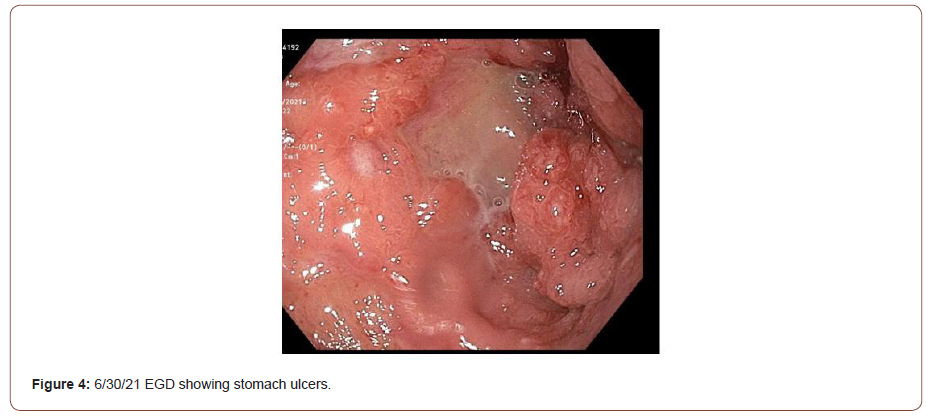
The patient continued to have large volume hematochezia prompting a repeat CTA that showed extravasation at the jejunum. In response, a small bowel enteroscopy was performed. Findings included numerous 1-5mm gastric nodules, a linear ulceration requiring mechanical clips, and many oozing duodenal ulcers (up to 10 mm) with adherent clots. During the patient’s third week of admission, his condition steadily deteriorated. Frequent hematochezia required massive transfusion protocol and vasopressor support. Despite two additional IR embolizations (proximal branch of inferior PDA, left gastric artery), overt bleeding continued from diffuse mucosal involvement. It was suspected that impaired intestinal mucosal integrity was causing infliximab to be lost through serum and stool (Figures 3 & 4). Therefore, IR placed a vasopressin infusion catheter at a branch of the superior mesenteric artery supplying the jejunum. Also, the decision was made to administer a second dose of infliximab 10 days after the first dose.
The significant number of blood transfusions led to volume overload and hypoxemia, requiring intubation. Intravenous and oral aminocaproic acid was initiated to inhibit fibrinolysis. A 1:1:1 transfusion strategy (i.e. packed red blood cells, fresh frozen plasma, platelets) was adopted to avoid diluting clotting factors. Recombinant factor VIIa was administered to counter bleeding. The hematochezia gradually improved, and the patient was weaned off catheter-directed vasopressin and other vasopressors. Transfusion requirements significantly improved 10 days following the second dose of infliximab. The patient was extubated after one week of mechanical ventilation.
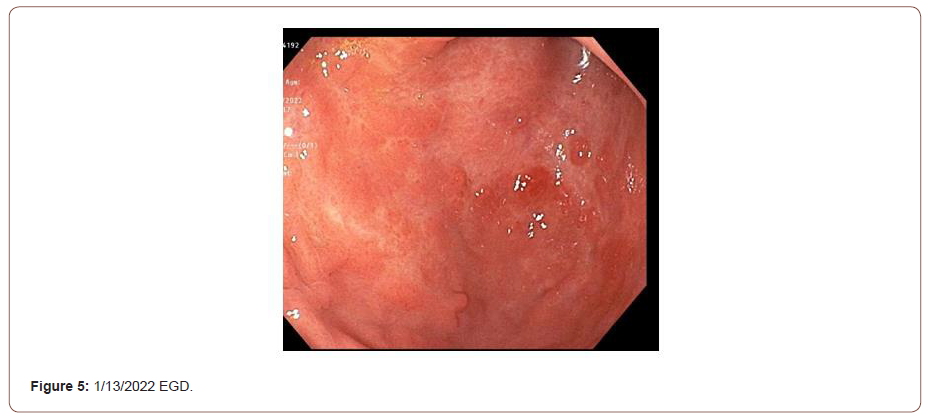
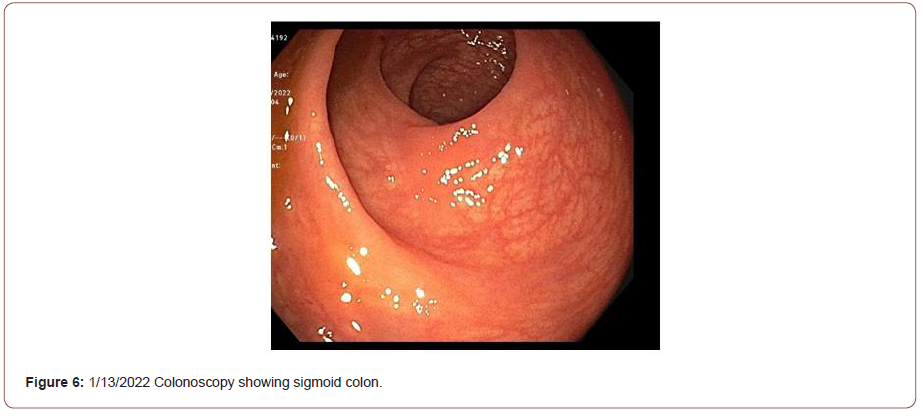
During the fourth week of admission, the patient’s clinical course improved, with no transfusion needs and a stable hemoglobin. In response to worsening leukopenia and thrombocytopenia, ganciclovir was replaced with foscarnet. The C-reactive protein improved from 78 to 49 mg/L. However, the infliximab serum level was undetectable with no detectable anti-drug antibody, a surprise given he had received 10 mg/kg dosing twice within the prior three weeks. A third dose of infliximab was given 21 days after the second dose. The patient continued to improve without overt GI bleeding, abdominal pain, or diet limitations. Before discharge, he received a fourth dose of infliximab (14 days after the third dose). After a few weeks in a physical rehabilitation center, the patient returned to his healthy baseline. A recent infliximab serum level was within the therapeutic range and EGD and colonoscopies showed healed mucosa (Figures 5 & 6).
Discussion
Approximately 0.6-4% of CD patients present with overt GI bleeding [1]. The novelty of this case lies in the severe, diffuse nature of his Crohn’s disease with large volume bleeding resulting in the loss of the initial doses of infliximab. The sub-therapeutic serum infliximab concentration delayed mucosal healing which in turn led to further gastrointestinal bleeding with life threatening consequences. In critically ill CD patients who present with large volume bleeding, it is imperative to assess infliximab levels and aggressively re-dose if needed. Fecal loss of infliximab in severe disease has been reported. Similarly, large volume enteral bleeding likely also impacts the serum infliximab level, necessitating more aggressive dosing of the drug.
Assessing serum levels of infliximab (or any Crohn’s treatment) is essential in patients with significant GI bleeding. Standard infliximab induction is 5 mg/kg at 0, 2 and 6 weeks [5]. In contrast, our patient received 10 mg/kg of infliximab on days 0, 10, 31, and 45. This aggressive regimen was used to counteract mucosal bleeding and resultant serum loss of infliximab. This loss was quantified when a serum infliximab level (after dose #2) returned as undetectable with no detectable anti-drug antibody. Losing biologics in stool during a flare has been documented, however, loss via serum has not. Therefore, an undetectable level of infliximab after 2 closely spaced 10 mg/kg doses was remarkable and unanticipated. In patients with severe IBD flares that appear refractory to biologic therapy, it is essential to check serum drug levels [6].
Proactive drug monitoring is crucial in patients presenting with severe disease, especially with significant blood loss. Custom dosing schedules of IBD treatments might be indicated in critically ill patients. Infliximab was initiated 1 month after our patient presented to a hospital, a delay that worsened mucosal disease. Especially given that our patient’s significant diffuse mucosal involvement precluded him from being a surgical candidate, prompt medical therapy was essential.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Podugu A, Tandon K, Castro FJ (2016) Crohn's disease presenting as acute gastrointestinal hemorrhage. World J Gastroenterol 22(16): 4073-4078.
- CCFA (2022) The Facts about Inflammatory Bowel Disease. Crohns Colitis Foundation.
- Jing Zeng, Feng Shen, Jian Gao Fan, Wen Song Ge (2022) Accelerated Infliximab Induction for Severe Lower Gastrointestinal Bleeding in a Young Patient with Crohn's Disease: A Case Report. World J Clin Cases 10(2): 733-740.
- Brandse JF, Vanden Brink GR, Wildenberg ME, Vander Kleij D, Rispens T, et al. (2015) Loss of Infliximab into Feces Is Associated with Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 149(2): 350-5.e2.
- Bryce K Perler, Ryan Ungaro, Grayson Baird, Meaghan Mallette, Renee Bright, et al. (2019) Presenting symptoms in inflammatory bowel disease: descriptive analysis of a community-based inception cohort. BMC Gastroenterol 19(1): 47.
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF (2006) The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55(6): 749-753.
-
Niharika Mallepally, Vijay Prabhakar, Sarah Sheibani and Jacques Van Dam*. Infliximab Redosing During Massive GI Bleeding Resulting from Severe Crohn’s Disease. Acad J Gastroenterol & Hepatol. 3(3): 2022. AJGH.MS.ID.000563.
-
GI Bleeding, Crohn’s Disease, Gastrointestinal hemorrhage, Vasopressors, Vasopressors, Abdominal pain, Leukopenia, Thrombocytopenia, Ganciclovir
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






