 Research Article
Research Article
Epidemiological Characteristics of Hepatitis B Positive Patients in Oman between 2009 and 2019: A Retrospective Cohort Study
Salah T Al Awaidy1*, Khalid Al Naamani2, Gowda Sathyanarayana3, Abdullah AlKalban2, BR Kamath2, Siham Al Sinani3, Elias Said4, Heba Omer5, AlZahraa Al Mehrzi2 and Ozayr Mahomed6
1Office of Health Affairs, Ministry of Health, Muscat, Oman
2Department of Medicine, Division of Gastroenterology and Hepatology. The Armed Forces Hospital, Oman
3Oman Medical Speciality Board, Oman
4Department of Microbiology and Immunology, College of Medicine and Health Sciences, Sultan Qaboos University, Oman
5Department of Endemic Medicine and Hepatology, Faculty of Medicine, Cairo University, Egypt
6Department of Public Health Medicine, University of KwaZulu Natal, Durban, South Africa
Salah T Al Awaidy, Office of Health Affairs, Ministry of Health, Muscat, Oman, Email: salah.awaidy@gmail.com.
Received Date: July 23, 2021; Published Date: August 09, 2021
Introduction
Introduction: The prevalence of chronic hepatitis B virus (HBV) in Oman was estimated to have been 2-7% prior to the introduction of vaccination. However, HBV remains a major concern among unvaccinated individuals, especially older adults.
Objective: The study aim is to describe the epidemiological characteristics of HBV positive patients at a specialized liver clinic at the Armed Forces Hospital, between January 2009 and December 2019. A retrospective cohort study was undertaken, and secondary data were obtained from electronic medical records.
Results: 593 patients with positive HBV serology were identified. Fifty-nine percent of patients were male, with a mean age of 42 years (SD: 10.05) compared to females (43 years, SD: 10.70). Eighty-five percent (492/580) of the patients were treatment naïve. Eighty-eight percent showed detectable HBV viremia. The median level of HBV DNA among chronic hepatitis BV patients who are HBeAg-positive was significantly higher: 1 884 847 IU/ml (IQR: 81662-486924145), as compared to the rest of chronic hepatitis BV patients: 339 IU/ml (IQR: 33.5-3366). Elevated ALT (≥ 40 IU/ ml) was found in 29% of the patients. liver abnormalities were detected by ultrasound in 28% (165) of patients. Treatment was introduced in 15% of patients.
Conclusion: The majority of HBV patients were seronegative for HB-Ag and were born before the vaccination era. Therefore, these patients with hepatitis B in dormancy do not need treatment, but regular monitoring and follow-up.
Keywords: HBV; Epidemiology; HBV DNA; HBe-Ag; Oman
Introduction
Viral hepatitis is a silent epidemic with serious impact on people, communities, and the overall health system. The World Health Organisation (WHO) launched the Global Health Sector Strategy on Viral Hepatitis 2016-2021 towards the Elimination of HBV and provisional guidance for country validation of viral hepatitis elimination in June 2021 [1]. In 2019, an estimated 296 million persons (3.8% of the population) were infected with the chronic hepatitis B virus (HBV) worldwide [1]. The World Health Organization (WHO) estimates the number of hepatitis B-related deaths in 2019 at 820,000 [450,000–950,000] [2]. The WHO estimates that about 3.3% of the people from the Eastern Mediterranean Region (EMR) are infected with chronic HBV where Yemen (12.7%-18.5%), Sudan (5%-8.2%) and Somalia (5.6%- 21.3%) are the most affected [3].
In Oman, prior to the introduction of vaccination (1989), the prevalence of chronic HBV infection was estimated at 2% to 7% [4]. Hepatitis B vaccine was administered 2, 4 and 6 months. The estimated average carrier rate for HBV was 2.5% (2.11-2.9%); and the number of people living with chronic HBV was modelled at 114,380 (96,850 - 32,931) [5]. A sero-prevalence study conducted in 2005 revealed that 15 years after the onset of HBV vaccination, chronic HBV sero-prevalence in children declined from 2.3% in 1990 to 0.5% in 2005 [6]. A study has found that HBV genotypes D and A are the most common in Oman [7]. In an earlier study, examining the characteristics of HBV infection in 154 Omani patients, for the majority of HBV-infected patients, the hepatitis B e antigen (HBeAg) was negative, and the hepatitis B viral load was low [8]. In Oman, despite the introduction of HBV immunization, chronic HBV remains a serious concern in the unvaccinated and the elderly. Little is known about the current cohort of HBV patients undergoing treatment in the country’s specialized liver clinic. This study is intended to describe the clinical and epidemiological features of a cohort of chronic HBV patients receiving care at a specialized liver clinic in Oman as part of HBV elimination strategy.
Method
Study design and population
This is a retrospective cohort study of all adult patients with chronic HBV infections who have been treated and followed up at the Armed Forces Hospital (AFH), Muscat, Oman, between January 2009 and December 2019. Data for all treated patients were recorded electronically and stored in the hospital database system. For the purposes of this study, only patients who have been diagnosed with chronic HBV at AFH and for which initial visit files were available and completed, were included in the study.
Study setting
The AFH is one of four tertiary hospitals in the capital city of Muscat. The hospital has a dedicated hepatology clinic, adequately equipped with all supportive equipments. This is one of three liver clinics within the country. An estimated 65 new cases of HBV are diagnosed each year. The liver clinic has a dedicated fibroscan machine and accepts patients from all governorates (provinces) of Oman.
Data Collection
Secondary data were collected from the hospital’s database system. The following variables were retrieved: socio-demographic (age, gender, smoking, alcohol history), hepatitis B serology including Hepatitis B surface antigen (HBsAg) and antibody (anti- HBs), Hepatitis B core antibodies (anti-HBc), Hepatitis B e antigen (HBeAg) and antibody (anti-HBe), Hepatitis B DNA (HBV DNA), and liver chemistry tests including bilirubin, albumin, transaminases (aspartate transaminase, (AST), and alanine transaminase, (ALT) and alkaline phosphatase, (ALP). Findings of ultrasound of the abdomen were also collected.
Statistical analysis
Numerical variables such as age and HBV DNA concentrations were analysed using centrally trended measurements. Categorical variables such as gender, radiological results and abnormalities in liver chemistry and hematology tests were calculated as proportions and displayed in frequency tables. Radiological results were classified as normal or reported abnormalities, liver chemistry tests and hematological tests were classified as normal or abnormal for laboratory reference ranges. A student t-test was used to identify likely differences between the mean age of men and women. Patients were classified as HBeAg positive chronic hepatitis B patients and having a high viral load with the HBV DNA levels > 20,000 IU/ml, and HBeAg negative chronic hepatitis B patients with HBV DNA levels > 2000 IU/ml [9].
Ethics and permission
Ethical approval was obtained from the Armed Forces Medical Services Ethics Committee. There was no contact with patients as the patients’ identifiers were coded; as a result, no informed consent was obtained. This study abides by the ethical principles of the Helsinki Declaration.
Result
Table 1:Socio-demographic and risk profile of chronic hepatitis B virus (HBV) and ultrasound findings cohort of patients, Oman, 2009-2019.

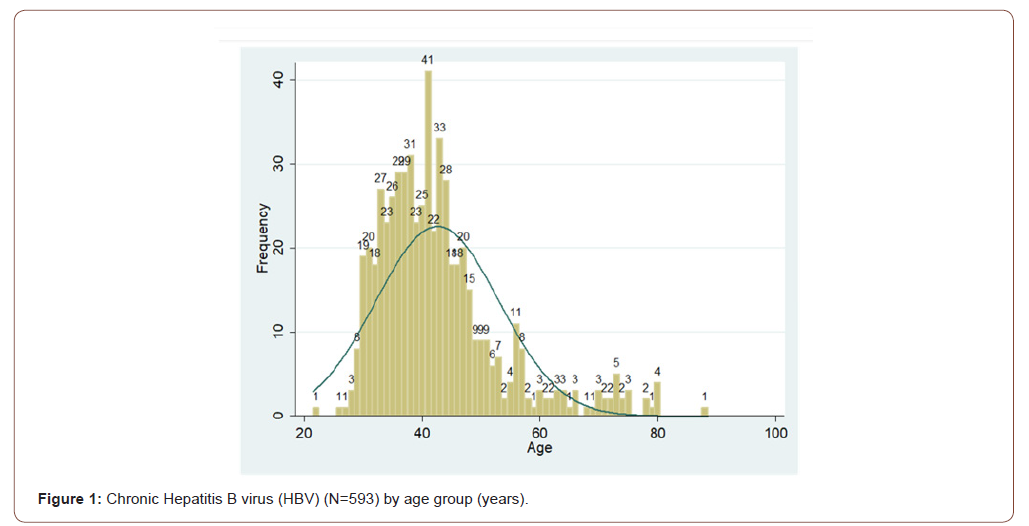
Data were extracted from initial visits to 593 patients with chronic HBV who patients that were assessed during the study period. All the cases were strictly treated in AFH hospital, Fifty-nine percent (352) of the patients were male. The mean age of the study population was 43 years (SD: 10.44), not statistically different from females with the mean age of (43 years SD: 10.70). Eighty-five percent (270) of participants were between 30 and 40 years old (Figure 1). Of the total, 0.5% were smokers, alcoholics consumed (1%), hypertensives (5%), diabetics (6%), and had a familial history of hepatitis B (24%). Family history of hepatocellular carcinoma (HCC) was present in (2 %) (Table 1).
Viral hepatitis profile
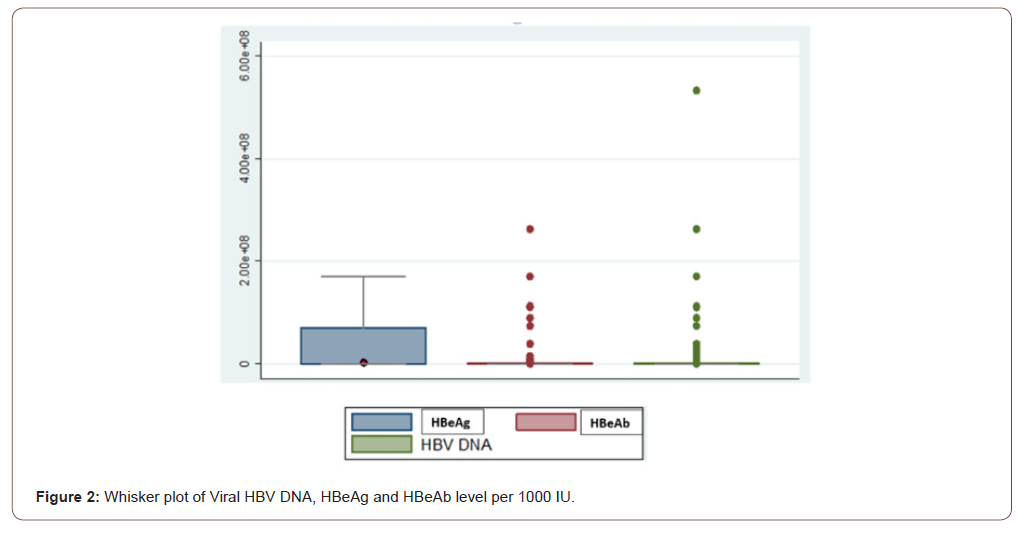
The initial cohort included 606 patients. Thirteen patients (2%) had anti-HBs and anti-HBc indicated HBV immunity due to previous exposure and were therefore excluded from the final analysis. Eighty-eight percent of patients (509) had detectable HBV DNA (>10 IU/ml). The median of HBV DNA level of the study population was 1239 IU/ml (QIC: 172-9324). Males had a greater median HBV DNA concentration (2046 IU/ml) than females (459 IU/ml). Ninety-four percent of patients (545) had HBeAg-negative chronic HBV. The median HBV DNA level for HBeAg- negative chronic HBV patients was 339 IU/ml (IQR: 33.5-3366). Ten percent (57) patients with HBeAg-negative chronic HBV were considered to have had a high viral load (HBV DNA > 2000 IU/mL). Six percent of chronic HBV patients (35) were HBeAg-positive. The median of HBV DNA level in these patients with HBeAg positive chronic hepatitis B was 1,884,847 IU/ml (IQR 81662-486924145). Twenty-nine (83%) of chronic HBV HBeAg-positive patients with chronic hepatitis B had a high viral load (HBV DNA > 20,000 IU/ml) (Figure 2).
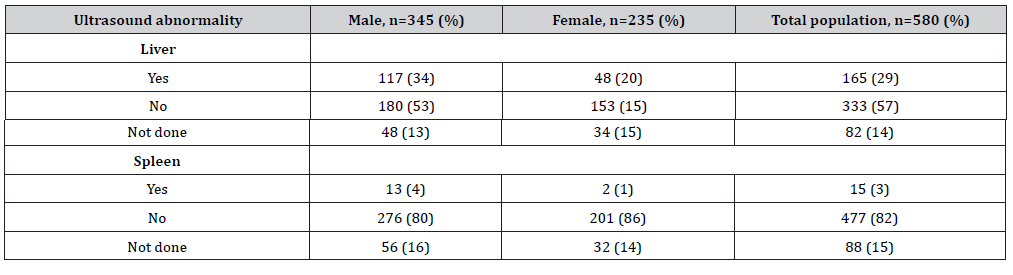
Table 2:Chronic hepatitis B virus (HBV) ultrasound profile of cohort of patients, Oman, 2009-2019.
Liver chemistry test abnormalities
Twenty-nine percent (167) of patients had high ALT (≥ 40 IU/ ml), while 14 percent (80) had high AST (≥ 40 IU/ml). The mean ALT levels for males (55.25 IU/ml) were not significantly different from that for females (51.45 IU/ml). Although the mean AST level for males were significantly higher (75.80 IU/ml) than for females (39.26 IU/ml), these differences were not clinically significant. The AST and ALT mean was deflected to the right because of numerous outliers.
Radiological abnormalities
During the initial consultation, 28% (165) of patients exhibited liver abnormalities detected by liver ultrasonography. This included hepatomegaly, altered liver echotexture, fatty liver and radiological characteristics of liver cirrhosis. Fifteen patients (3%) had splenomegaly, indicating portal hypertension (Table 2).
Treatment status
Eighty-five percent (492/580) of the patients were treatment naïve. Of the 15% (88) treated patients, 9% used tenofovir, 4.3% used entercavir, 1% used adenovirus and 0.7% used lamuvidin.
Discussion
HBV infection is still a major concern in Oman. The mean age of the patients in this study was 43 years (SD: 10.44) and the median age was 41 years (IQR: 36-46). The majority of the patients were between 30 and 40 years of age (55%) signifying that these patients had missed the immunization program, and as such did not receive the birth dose (within 24 hours) as this was introduced back in 2008 [6] or were born to HBV infected mothers. Another factor that could have contributed to a higher rate of infection among this group is the length of time the follow-up vaccination campaigns took to cover the entire country, thus increasing the risk of unvaccinated children contracting the infection from infected family members [10]. The Centers for Disease Control and Prevention (CDC, USA) recommended that the screening of pregnant women for HBV is the standard of care, regardless of previous testing or vaccination [11]. This screening would identify patients that might require treatment as well as infants who require prophylaxis, and other individuals who are in contact with infected people and who would also benefit from testing, counselling, vaccination and therapy, if needed.
Patients in the current study (mean age 43 years) were relatively young. In a study from neighbouring Saudi Arabia the mean age, among admitted patients was (46.9 ± 14.3 years) [12], however, in another study from Pakistan the mean age in a tertiary hospital 7 was (29.86 years, SD: 13.68). The young age of the Pakistani study might be due to the strength of the country-based screening program [13]. The current study demonstrates a male majority (59%), displaying similar results to another hospital based retrospective study of 5 years at a tertiary level hospital in Saudi Arabia that showed a 60% prevalence in males. Similarly, a prevalence of 69.23% among men was recorded in Kerala, India. Further studies in Pakistan found a male prevalence of 79.5% and 68.15%, respectively [14,15].
The prevalence of chronic HBeAg-positive in the current study is similar to a prospective cohort study amongst 1158 Native Americans throughout Alaska who were tested serially for HBeAg for a median of 20.5 years [16]. However, the prevalence of HBeAg-positive in our study, although high, is much lower than in other countries. In a study from from Nigeria, 19.2% of screened chronic HBV individuals were found to be HBeAg-positive [17]. Furthermore, a number of studies have shown that HBeAg-positive chronic hepatitis B tend to have high levels of HBV DNA that were associated with increased risk of cirrhosis and HCC [18-20]. In this study, 29% of HBeAg-positive patients had a high HBV DNA with a similar potential implication on prognosis. The majority of the patients were HBeAg-negative with low HBV DNA. This is consistent with previous studies from other parts of the world that have demonstrated low levels of HBV DNA in low level of HBVDNA in HBeAg-negative chronic hepatitis B patients [21]. Those patients who are HBeAg-negative and have low HBV DNA are at lower risk of progression to cirrhosis, or HCC [21].
Fifty-seven (11%) of the patients who are HBeAg-negative had high viral load (> 2000 IU/ml). This high level of HBV DNA among HBe-Ag negative patients that could be attributed to the presence of pre-core and core mutations, may render them vulnerable to develop advanced hepatic fibrosis [22,23]. Nearly one-third of our cohort had a high ALT level of ≥ 40 IU/ml. Historically, the upper limit of normal (ULN) for ALT in males and females was 40IU/ ml. Later studies evaluate the ULN for ALT in both genders among healthy particpants after excluding common cause of elevated transaminases such as non-alcoholic steatohepatitis (NASH), alcohol consumption and chronic HCV infection. The current ULN for ALT in adult men is 30IU/ml and 19 IU/ml in women [24].
Elevated transaminases are used by all international guidelines [25,26] to assess disease activity and therefore is an indication for treatment in addition to other indices. A minimum of two times ULN is required for treatment following exclusion of other causes of transaminitis [25,26]. A recent study, looking at a large number of patients (2991) with chronic HBV and correlating ALT level with liver histology concluded that an ALT level less than twice ULN is associated with a low probability of significant inflammation [27].
Study limitation
The design of our study predisposes to a number of limitations. First, secondary data were obtained. Thus, the integrity of the results is dependent upon the accuracy and integrity of the data. For approximately 8% of the patients, there was initially no information on HBV DNA. Secondly, we did not collect information on risk factors associated with acquiring hepatitis B infection. Finally, the clinical data were not collected.
Conclusion
This study forms the basis of new research into chronic HBV infection in Oman. It indicates that that the introduction of childhood vaccination had significantly impacted the rate of HBV infections in Oman as most of infected patients are among males. Most of the patients were HBeAg-negative chronic hepatitis HBV patients with low HBV DNA and required no treatment but longterm follow-up. Further studies are warranted and could include case-control studies assessing risk factors associated with cirrhosis and/or HCC.
Author Contributions
KA, STA, GS, AA, BRK, SA, ES, HO and AA conceptualized the project and conducted initial background research, STA, OM and KA planned and conducted the formal analysis and validation, STA, OM and KA interpreted the results, STA led the writing (original draft and editing) of the manuscript, and STA, KA and OM supervised the project. All authors approved the final article.
Acknowledgement
The authors wish to acknowledge all the participants who were instrumental in the development of the manuscript namely Mamoun Elsheikh and Magda Al Wahabi.
Conflicting Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Navarro VJ, Belle SH, D Amato M, Adfhal N, Brunt EM, et al. (2019) Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PloS one 14(9): e0221683.
- Farias LGN, Oliveira AF, Teles HCC, Santana DS, Oliveira SL, et al. (2016) Efeitos da esteatose hepática não alcoólica nas variáveis hemodinâmicas dos vasos do fígado através da dopplerfluxometria. Capa V. 28(18).
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. (2016) Global epidemiology of nonalcoholic fatty liver disease-meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1): 73-84.
- Santana JT, Mota AVH, Gonzaga YHG, Gomes RMOP, Melo LC, et al. (2021) Perfil metabólico e antropométrico dos pacientes obesos e não obesos portadores de esteatose hepática não alcoó Revista Eletrônica Acervo Saúde 13(2): e5525-e.
- Vespasiani-Gentilucci U, Gallo P, Dell’Unto C, Volpentesta M, Antonelli-Incalzi R, et al. (2018) Promoting genetics in non-alcoholic fatty liver disease: Combined risk score through polymorphisms and clinical variables. World J Gastroenterol 24(43): 4835-4845.
- Mesquita AO, Cardoso EF, Fernandes LG, Souza VM, Rebelo BRR, et al. (2019) Esteatose hepática não alcoólica: uma análise dos seus fatores de risco. RESU – Revista Educação em Saúde V. 7(1).
- Mendonça TM, Mendonça LM, Noronha VFCM, Mota AVH, Silva Filho RC, et al. (2020) Hiperferritinemia em pacientes com doença hepática gordurosa não alcoó Brazilian Journal of Health Review 3(4): 10833-10843.
- Lankarani KB, Ghaffarpasand F, Mahmoodi M, Lotfi M, Zamiri N, et al. (2013) Nonalcoholic fatty liver disease in southern Iran: a population based study. Hepat Mon 13(5): e9248.
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, et al. (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1): 328-357.
- Pedrassa BC, Rocha EL, Kierszenbaum ML, Bormann RL, Torres LR, et al. (2014) Uncommon hepatic tumors: iconographic essay-Part 1. Radiol Bras 47(5): 310-316.
- Wong VWS, Chan WK, Chitturi S, Chawla Y, Dan YY, et al. (2018) Asia–Pacific Working Party on Non‐alcoholic Fatty Liver Disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 33(1): 70-85.
- Solhi H, Ghahremani R, Kazemifar AM, Yazdi ZH (2014) Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Caspian J Intern Med 5(1): 9-12.
- Hajagha MA, Ziaei A, Rafiei R (2008) The efficacy of silymarin in decreasing transaminase activities in non-alcoholic fatty liver disease: A randomized controlled clinical trial.
- Zhong S, Fan Y, Yan Q, Fan X, Wu B, et al. (2017) The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine 96(49): e9061.
- Cachia R, Compañó R, Da Costa O (2007) Grasping the potential of online social networks for foresight. Technological Forecasting and Social Change 74(8): 1179-1203.
- Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, et al. (2012) Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med 52(9): 1658-1665.
- Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F (2013) Silymarin in non-alcoholic fatty liver disease. World J Hepatol 5(3): 109-113.
-
Salah T Al Awaidy, Khalid Al Naamani, Gowda Sathyanarayana, Abdullah AlKalban etc all,... Epidemiological Characteristics of Hepatitis B Positive Patients in Oman between 2009 and 2019: A Retrospective Cohort Study. Acad J Gastroenterol & Hepatol. 3(1): 2021. AJGH.MS.ID.000553.
-
Chronic hepatitis, HBV, Epidemiology, HBV DNA, HBe-Ag, Liver clinic, Hematology, Hepatic fibrosis, Hypertensives, Diabetics
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






