 Research Article
Research Article
Efficacy and Safety of Esolgafate, A Pre-Polymerized Cross-Linked Sucralfate Medical Device for NERD. A Randomized Double-Blind Placebo-Controlled Trial
Ricky Wayne McCullough1,2*
1Translational Medicine Clinic and Research Center, USA
2Department of Internal Medicine and Emergency Medicine, Warren Alpert Brown University School of Medicine, USA
Ricky Wayne McCullough, Translational Medicine Clinic and Research Center, Storrs Connecticut, USA.
Received Date: April 13, 2020; Published Date: April 22, 2020
Abstract
Background: Unlike those with erosive reflux disease, patients with non-erosive reflux disease fail to adequately respond to proton pump inhibitors (PPI’s). Pre-polymerized sucralfate barrier therapy (PPSBT) recognized by US FDA as a medical device has significantly enhanced mucosal bioadherence compared to standard sucralfate drug.
Aim: To evaluate whether enhanced mucosal protection by PPSBT can provide relevant symptom relief for NERD compared to placebo, even in undifferentiated population of NERD patients.
Methods: In a multi-center randomized double-blind placebo controlled trial 42 patient with NERD were randomized to receive Esolgafate, a pre-polymerized cross-linked sucralfate barrier therapy or placebo. No pH monitoring was conducted to determine representative proportion of the 3 sub-types of NERD. Antacids were available to each group as rescue medication. Symptoms of heartburn, reflux sensation, retrosternal discomfort were evaluated before and after treatment. Adverse events were assessed.
Results: At the end of the trial, for patients taking PPSBT primary endpoints were met in 90% for heartburn, 83.3% for reflux sensation and 88.2 % for retrosternal discomfort compared to 11.1%, 25% and 20% for those using placebo (p <0.01).
Conclusion: The barrier effect of Esolgafate suggests that enhanced mucosal protection by PPSBT alone could improve symptom control in NERD patients undifferentiated by sub-type of non-erosive heartburn.
Keywords: Polymerized sucralfate; NERD; Barrier therapy; Sucralfate suspension
Introduction
Gastric refluxate into the lower esophagus is common and physiologic, occurring in the vast majority of people and is often asymptomatic [1,2]. However, symptomatic presence of gastric reflux is heralded by heartburn and is subsequently referred to as gastroesophageal reflux disease or GERD. Patients with heartburn and no prior history of GERD can be subdivided by endoscopy into two main cohorts - those with mucosal erosions or erosive GERD and those without erosions or non-erosive GERD [3]. With the introduction of Rome IV [4], the latter group of non-erosive heartburn, can be further differentiated into three clinical sub-categories based on frequency of acid reflux events and the association of symptoms with reflux events [5]. Functional heartburn is non-erosive heartburn with normal frequency of acid exposure but negative symptom reflux association. Reflux hypersensitivity syndrome is non-erosive heartburn with normal frequency of acid exposure but positive symptom association with reflux events. Non-erosive heartburn with above normal frequency of acid exposure that is positively associated with reflux events [6] is referred to as NERD, by Rome IV criteria - nonerosive reflux disease [4]. Besides heartburn and reflex sensation, retrosternal chest discomfort or pain can accompany any of the three Rome IV sub-categories of NERD. Most publications prior to the Rome IV differentiation have regarded NERD as any syndrome of recurring heartburn associated with an erosion-free mucosa [7]. As a whole, the pre-Rome IV heterogeneous population of NERD patients had chronic recurring heartburn, reflux sensation and chest discomfort. As a group, they are minimally responsive to acid-controlling therapies such as proton pump inhibitors (PPI), histamine-2 receptor antagonists (H2RA), and antacids. Consequently, significant clinical effects using acid-controlling therapies require long term chronic treatment spanning 26 to 52 weeks [8]. Long term therapy using PPI’s or H2RA’s are associated with adverse events, such as headache, diarrhea, abdominal pain, and breakthrough nocturnal heartburn.
Heartburn, based on afferent innervation of the esophagus by the vagus nerve, is a shared characteristic of each Rome IV patient sub-cohort of NERD [9]. Heartburn sensation arises from firing of sensory neurons in the distal esophagus and signals a histochemical disturbance within the mucosa. Histochemical disturbances within the esophageal mucosa excite submucosal pain receptors (called nociceptors) [10] and upregulate afferent neurons. Afferent neurons, coursing beneath the esophageal epithelium with the lamina propria, are equipped with voltage gated receptors - acid sensing ion channels (ASIC) [11] and the transient receptor potential vanilloid receptors (TRPV) [12]. These voltage-gated nociceptors require transmucosal ion flux (exchange positive and negative ions) which keep afferent neurons switchedon in NERD patients experiencing heartburn, reflux sensation and retrosternal discomfort or pain. Thus, the lack of symptomatic responsiveness to PPI’s, H2RA’s and antacids in these patients simply involve histochemical processes that are exclusive of the mucosa’s reaction to pH. Observations that pH-responsive and pHnon- responsive NERD patients exhibit dilated intercellular spaces in the esophageal epithelium [13] and that these patient cohorts have basal cell hyperplasia not seen in controls [14], imply that the mucosal reaction specific to NERD patients is likely not a problem of acid control or incorrect pH of gastric refluxate.
Gastric refluxate is a backwash of mineral acid containing dissolved bile acids and serine proteases. Mineral acid (hydrochloric acid) and bile reflux together [15,16]. In patients with symptomatic GERD reflux events containing bile may be more numerous than reflux events of acid alone [17]. Dissolved bile acids, particularly taurine conjugates of cholic acid and chenodeoxycholic acid, [18,19] are not deterred by acid-controlling therapies, and there is evidence to suggest, that there may be facilitation [20]. Both mineral acid and dissolved bile acids can co-dependently and independently initiate histochemical mucosal events responsible for NERD symptoms. Hints of a pro-inflammatory immunologic process have been previously reported [21-24] and recently confirmed by in vivo and in vitro observations.
The data reported here was first presented in abstract form at a previous Digestive Disease Week of the American Gastroenterological Association and addresses the clinical efficacy and safety of a suspension form of pre-polymerized sucralfate barrier therapy (PPSBT). As with all sucralfate-based therapies, PPSBT is non-systemic, site specific and cytoprotective. It is prescribed to function as a physical barrier to gastric refluxate. Limiting access of gastric refluxate to esophageal mucosa should diminish refluxate-mediated mucosal inflammation invisible to the eye but responsible for symptoms of heartburn, reflux sensation and chest discomfort in patients with NERD.
Methods
Objectives and hypotheses
The main objective of this trial was to assess the effectiveness and safety of pre-polymerized sucralfate barrier therapy suspension in the treatment of non-erosive reflux disorder. In this trial placebo was used as a comparator which is optimal in testing the efficacy of a new treatments [25].
Ethics and trial registration
The trial was registered at Medical Research Council of Bangladesh (BMRC) [26] who provided institutional review of its protocol. Patients provided written informed consent to participate in the trial in accordance with the Declaration of Helsinki.
ISO 14155 compliant clinical investigation of medical device
For scientific transparency in the evaluation of medical devices [27], the current study was designed to be compliant with current ISO 14155 standards which addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations carried out in human subjects [28]. The current trial assesses the safety and performance of Esolgafate as a barrier therapy medical device for NERD. Safety of sucralfate-based products are well documented and has been established since 1968 [29]. Identified are rules and procedures of data collection, statistical power of the study and rationale of sample size. Recruitment of participants, randomization, concealment, and allocation of interventions were performed in a manner to minimize bias, ensure collection of objective and credible data, and support the overall goal of protecting patients’ safety and well-being.
Study design
The study was designed as a randomized double-blind placebocontrolled trial with allocation ratio 1:1. Each of the two study arms received identically marked bottles containing a white suspension identical in color and flavor. One intervention contained prepolymerized sucralfate barrier therapy (PPSBT) suspension, while the other, placebo, contained no sucralfate.
Setting and participants
Recruitment of participants took place in three medical center clinics where patients received primary and specialty medical care services. Eleven gastroenterologists conducted patient recruitment.
Inclusion criteria
Inclusion criteria for male or females over the age of 18 years, included dyspeptic symptoms, specifically heartburn, reflux sensation and retrosternal discomfort occurring 3-7 times per week for 3 months leading up to the study with endoscopic evidence of no mucosal erosions by Hetzel-Dent grading system [30]. A Hetzel- Dent Grade of 0 or 1 was required for inclusion. Included patients demonstrated ability to follow protocol instructions and complete self-administered questionnaire. All participants had the right to withdraw from the study at any time with no obligation to give reason for their decision.
Exclusion criteria
Individuals with erosive gastroesophageal reflux disease (eGERD) were excluded. Those with difficulty following protocol instructions (diary maintenance, keeping follow-up appointments etc) were excluded. Patients with Barrett’s esophagus, peptic strictures, peptic ulcer disease or requiring motility altering drugs were excluded.
Randomization criteria
Participants were assigned by simple randomization into one of 2 groups, PPSBT or placebo. Simple randomization was aided by use of a random number table wherein patients who had been assigned a two digit number from 01 to 70 plus were sequentially assigned to one of two treatment groups based on the first digits of the random number table being either even or odd.
Interventions
The intervention under investigation include suspension of pre-polymerized cross-linked sucralfate (PPSBT) barrier therapy and placebo which was identical to PPSBT in color, favor and ingredient but contained no sucralfate. Both were manufactured by Pharmaco Laboratories Limited for Mueller Medical International (MMI). Each intervention was dispensed by a single hospital pharmacy associated with the medical centers responsible for recruitment. The pharmacology of PPSBT is relatively new and differs significantly from non-polymerized sucralfate. The latter form of sucralfate is regulated as a drug because of required in situ polymerization by gastric acid following ingestion of biologically inert sucralfate. Polymerization and cross-linking process for PCLS involves use of cationic organic acid. It is therefore more resistant to hydration than sucralfate polymerized by gastric acid. Three hours post-administration, PCLS achieves and maintains a surface concentration of sucralfate that is 7 fold (or 800%) greater on normal mucosa and 23 fold (or 2400%) greater on acid-injured mucosa compared to concentrations achieved by gastric-acid polymerized sucralfate [31]. Dosing volume for each intervention was 15ml taken twice daily for 28 days. This volume of PPSBT contained 1.5grams of sucralfate while the same volume of placebo contained no sucralfate.
Concomitant medications
Except for the use of antacids, participants were not permitted use of any anti-peptic. Two 300ml bottles of aluminum hydroxide/ magnesium hydroxide (400mg/400mg per 10ml) were provided to each participants every 7 days of the 28 day trial whether or not antacids were used. All used and unused bottles of antacids were collected at completion of trial.
Allocation concealment and blinding
Consecutive randomization numbers were given to participants and the study interventions were packaged and assigned consecutive numbers according to the randomization list from which participant numbers were generated. Bottles distributed to participants contained either pre-polymerized sucralfate barrier therapy (PPSBT) suspension or the suspension vehicle for PPSBT without sucralfate. Each intervention was identical in color and flavor. Participants, outcome assessors and person responsible for the statistical analysis were blinded to the intervention until completion of the study. Personal information about potential and enrolled participants was accessible only to the researchers involved in the trial.
Compliance
Participants were required to bring any remaining study product (including empty bottles) and diary to the study site at the end of the intervention period. Compliance with the study protocol was checked by counting the number of bottles left unused. Generally, participants receiving less than 75% of recommended doses would be considered noncompliant [32].
Primary outcome
The primary outcome was the relief of heartburn, reflux sensation and retrosternal chest discomfort. The number and severity of each symptom episode were recorded by participant in a dairy as the episode occurred. Severity was assessed by a patientgraded symptom intensity tool having a 5 point Likert scale, where 0 = absence of symptom, 1 = minimal awareness of symptom, easily tolerated, 2 = awareness of symptom, bothersome but tolerable without impairment of daily living or sleep, 3 = very bothersome interfering but not impairing daily living or sleep and 4 = intolerable and impairing daily living or sleep.
A quantitative score for heartburn (HB), reflux (RR) and retrosternal discomfort was calculated by multiplying the number of such episodes per week by the severity on the Likert scale. Examiners averaged the resultant quantities on a per day basis to arrive at a daily symptom specific score. The quantitative analysis was performed at end of Week 0, 1, 2, 3 and 4. An outcome was deemed positive if there was 50% improvement in the daily total symptom scores (a multiple of daily symptom frequency and symptom severity) in 28 days of the trial compared to total symptom scores of Week 0.
Secondary outcome
There were two secondary outcomes. One involved reliance on concomitant use of antacids as measured by count of used and unused antacid bottles assessed on a weekly basis. Whether required or not, two 300ml bottles of aluminum hydroxide/magnesium hydroxide antacid (400mg/400mg per 10ml) were provided per participant per week at the beginning of Week 1,2,3 and 4. At end of each week patients returned used and unused bottles of antacids for examination and a replacement pair of bottles were supplied. The second secondary outcome was emergence of adverse events.
Power calculation
Table 1:Facial functions of the patients before the treatment.
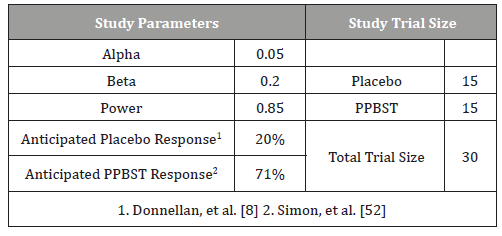
Table 1 provides parameters for sample size calculation. The primary outcome of the trial is symptomatic relief of NERD. Based on the Cochrane meta-analysis involving medical treatments for endoscopic negative reflux disease [8] a placebo response of 20% was selected. Simon, et al. [33] using twice daily dosing of concentrated sucralfate gel over 42 days achieved symptomatic relief of NERD in 71% of 70 patients. Given that PPSBT is a suspension and the trial is for 28 days, to calculate an appropriate sample size, an anticipated response rate was set at 71%. Thus to detect similar differences in this trial, a placebo rate of 15% and PPSBT rate of 71% wherein the alpha error is 0.05 and beta error is 0.2 for a power of 0.85, then the sample size for the study should be 28 participants with 14 participants per treatment arm. Assuming a 20% loss to follow-up and the incidence of erosive GERD among patients with dyspepsia, 72 participants were recruited.
Statistical analysis
All analysis was conducted on an intention-to-treat basis, including all patients in groups to which they were randomized for whom outcomes would be available (including withdrawals and lost to follow-ups). Descriptive statistics were used to summarize baseline characteristics. The Student t test was used to compare mean values of continuous variables for approximating a normal distribution. The chi-square test was used to compare percentages. The difference between study groups was considered significant with the p value was less than 0.05, when 95% CI for RR did not include 1.0 or when the 95% CI for mean difference did not include 0. All statistical tests were two tailed and performed at the 5% level of significance.
Result
Conduct of the trial
Patient flow in trial is shown in Figure 1. A total of 72 patients with dyspepsia recruited by gastroenterologists were assessed for trial eligibility. Of these 6 declined to participate, and 5 did not meet inclusion criteria due to experiencing only 1 or 2 of the required three symptoms once to twice weekly over the preceding 3 months. Of the 61 patients who underwent upper endoscopy, 19 had a Hetzel-Dent Grade greater than 1, or erosive GERD. The remaining 42 patients with NERD were randomized to 1 of 2 treatment arms.

Adequacy of sample size
The study was appropriately powered. Based on the statistical power applied to trial design, 17 participants per treatment arm were required. Each treatment arm exceeded the statistically required number, with PPSBT group having 20 patients and placebo arm with 18.
Participants
Patients randomized into two treatment arms were comparable in age, gender, days per week of heartburn, reflux, and retrosternal chest discomfort (Table 2). Patients in PPSBT treatment arm suffered from NERD for an average of 4.2 ± 2.2 years, while those in placebo arm suffered from NERD for 3.6± 2.2 years. The PPSBT and placebo group experienced heartburn, reflux, and retrosternal discomfort roughly the same frequency, 100% and 100%, 90% vs 88.8% and 85% versus 83.3%, respectively. Prior to trial participants were treated with a PPI or PPI plus antacids. Patients were similarly matched for presence of hiatal hernia, helicobacter pylori, and Hetzel-Dent score of 0 and 1. Helicobacter pylori was assessed by endoscopic biopsy from the antrum near pyloric ring and along the greater curvature of the corpus.
Table 2:Baseline Characteristics of Randomized Patients.
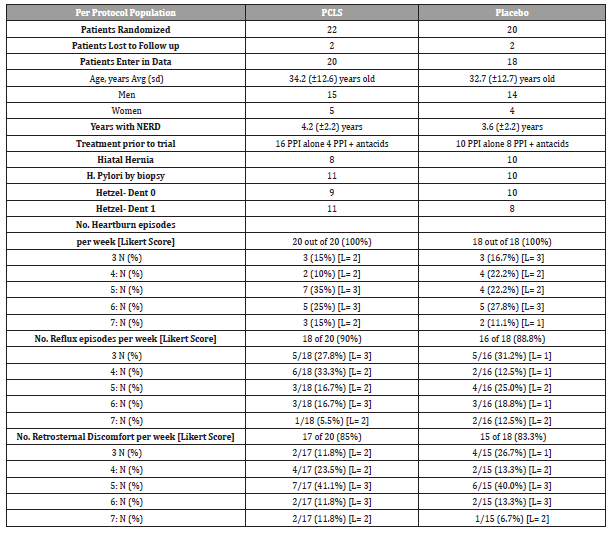
As shown in Table 2, while the majority of NERD symptoms could be sorted into three categories (heartburn, reflux sensation, retrosternal discomfort), not all patients exhibited each category equally. While 100% of patients in PPSBT and placebo groups had heartburn, 90% and 85% of PPBST and 88% and 83% of placebo patients experienced reflux sensation and retrosternal discomfort, respectively. An outcome was deemed positive if there was 50% improvement of the daily total symptom score which is a multiple of daily symptom frequency and symptom severity (Likert scale) over 28 days of the trial.
Compliance of the trial
Compliance across all treatment arms was acceptable for both PPSBT (90.9%) and placebo treatment arm (90%) due in large part to 4 patients lost to follow up, 2 from PPSBT group and 2 from the placebo group.
Safety and adverse events
All patients took multiple doses of each intervention and were included in the safety analysis. Results are presented for the intent to treat population. No patient reported any adverse reactions to either PPSBT or to placebo.
Primary outcome
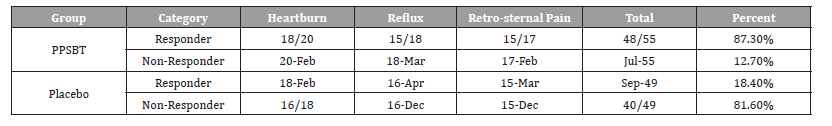
Table 3 shows 28 day symptomatic relief of three categories of NERD dyspepsia using PPSBT and placebo. A positive outcome was achieved by 50% improvement of daily total symptom score at the end of 28 days. The dependence of symptom improvement using PPSBT was evaluated (Table 4). The relief of heartburn, reflux sensation and retrosternal pain in patients with NERD using PPSBT versus placebo was 90% vs 11% for heartburn, 83.3% vs 25% for reflux sensation and 88.2% vs 20% for retrosternal pain. The Yates corrected chi-square values between treatment groups were 121.7, 65.4 and 90.4 respectively with p-value <0.00001 at significance level of p<0.01. The composite of NERD symptom response (Table 5) showed an overall NERD symptom response of 87.3% for PPSBT versus 18.4% for placebo, with Yates corrected chi-square value of 92.7, p-value <0.0001 at significance of p<0.01.
Table 3:Symptomatic Outcome.
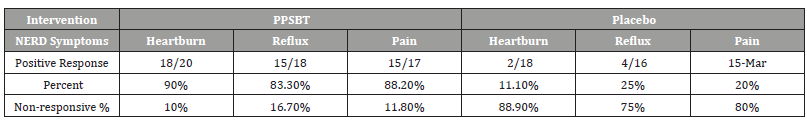
Table 4:Statistical Difference in Outcomes for Interventions.
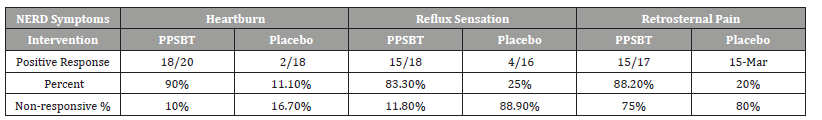
Table 5:Composite of Overall NERD Symptom Response.
Secondary outcome
Adverse events: There were no observed or reported adverse events in the trial.
Dependence on antacids: The dependence of symptom relief on the use of antacids in patients using PPSBT and placebo was evaluated (Table 6). Placebo was associated with a high use of antacid by patients, presumably to mitigate NERD symptoms.
Table 6:Uses of Antacid During Trial.
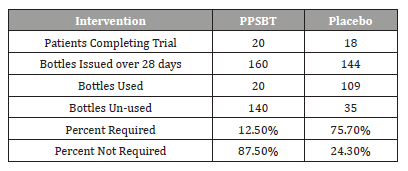
As previously stated, two 300ml bottles of aluminum hydroxide/magnesium hydroxide antacid (400mg/400mg per 10ml) were provided per participant at the beginning of Week 1,2,3 and 4, whether required or not. At end of each week patients returned used and unused bottles of antacids for examination and a replacement pair of bottles were supplied.
As seen in Table 6, 75.7% of supplied antacid was required bythose in placebo group, compared to 12.5% of supplied antacid required in PPSBT group. The Yates corrected chi-square was 77.8 with p-value < 0.00001 at significance of p<0.01.
Discussion
This study on the symptomatic relief of non-erosive GERD was a 28 day randomized placebo-controlled double-blind trial assessing the efficacy and safety of pre-polymerized sucralfate barrier therapy. Efficacy was assessed by quantifying symptomatic relief of heartburn, reflux sensation and retrosternal chest discomfort in patients with NERD. PPSBT is a medical device requiring any trial to be ISO 14155 compliant. The clinical investigation of PPSBT was properly powered having appropriate sample sizes per treatment arm. In fact, had the overall observed outcomes for placebo (18.4%) and for PPSBT (87.3%) been used to calculate the sample size, the required population size for the trial would have been 16, or 8 patients per treatment arm at a power of 0.85 with an alpha of 0.05. This trial had 38 patients, 20 patients in the PPSBT treatment arm and 18 in placebo arm, with no less than 15 patients per symptom cohort of either treatment arm. The trial was conducted transparently with no significant compliance issues among participants. The defining traits in patient population of each treatment arm (gender, age, years with disease, presence of H. pylori, hiatal hernia, endoscopic findings) were roughly equivalent. Therefore, by ISO 14155 standards meaningful conclusions on outcomes can be made.
This randomized double-blind placebo controlled trial on the efficacy and safety of pre-polymerized sucralfate barrier therapy found it to be safe and effective in patients with symptomatic NERD. Patients with non-erosive heartburn are a heterogeneous group of patients with normal to abnormal frequency of reflux events per 24 hours associated with varying degree of responsiveness to reflux events. Functional heartburn, reflux hypersensitivity and frequent symptomatic reflux that cause heartburn populate the heterogeneous Rome IV clinical group generally known as NERD. This heterogeneity is a challenge for treatment options, most of which rely on controlling pH. Following 28 days of maintenance dose versus healing (usually double) doses of PPI’s, patients with erosive GERD show a dose dependent rate of response. Patients with NERD do not [34]. Compared to those with erosive GERD, there can be up to a threefold lag in NERD patients in their response to PPI’s. Since pH monitoring for symptomatic reflux was not conducted in this current trial, the proportion of patients with functional heartburn, reflux hypersensitivity or pure NERD was not known. Epidemiologically it is reasonable to assume that each type were present in this trial, though this could not be verified to any degree of certainty. NERD patients in this trial were not differentiated by sub-cohort and likely were representative of the heterogeneity common to this clinical population.
Yet regardless of their heterogeneity, there are shared physiologic characteristics among NERD patients, which include: (a) impaired mucosal integrity with low basal impedance and slow recovery time following acid challenge [35]; (b) dilated intercellular spaces [13]; (c) basal cell hyperplasia beyond healthy controls [14]; (d) exhibit low grade inflammation heralded by pro-inflammatory cytokines [21,24,36,37]; (e) nearly double the number of submucosal mast cells in the lower esophagus with a near three-fold increased percentage of them degranulated [38]; (f) elevated submucosal presence of nerve growth factor; and (g) increased expression of TRPV1 on afferent neurons [39,40]. The singular event giving rise to these shared characteristics is repeated backwash of gastric refluxate onto the esophageal mucosa. To biomedical purists, physically denying access of gastric refluxate to the shared characteristics of this heterogeneous clinical group is the the action of choice. Fundoplication is effective in NERD as well as GERD in limiting access of gastric refluxate [41], yet it remains an alternative only in the event of failure of less invasive measures.
Alginate-containing therapies form rafts of amalgamated calcium cross-linked polysaccharide that cap off and restrict gastric contents to the stomach but does not coat the esophagus. In contradistinction to alginate’s supra-gastric/infra-esophageal raft formation, is sucralfate’s mechanism of mucosal coating. It is a site specific muco-adherent deposition of polysulfate saccharide that electrostatically interdigitates with mucin overlying the esophagus [42-44]. In animal models (rat and pigs) and other studies, sucralfate engagement strengthens the integrity of mucin in a dose dependent fashion [45] resulting in a multifold enhancement of viscosity, 60% increase hydrophobicity which in turn causes decreased permeability of hydrogen ions (acid) [46], of cations (e.g., calcium) [47], of bile acids [48] as well as an inhibition of endogenous proteases (pepsin) [49] and exogenous (H. pylori) mucolytic proteases [50,51]. Sucralfate biophysically deny irritants access to esophageal mucosa. Sucralfate is less an anti-reflux and more anti-access therapeutic option. Its clinical effectiveness increases with its surface concentration.
Earlier efficacy of sucralfate for symptomatic NERD has been rarely reported. Simon, et al. [33] using a sucralfate gel twice daily dosing over 42 days achieved symptomatic relief of NERD in 71% of patients. Gel formation enhances the surface concentration of sucralfate by 200% compared to equimolar amounts of nongel sucralfate (suspension form) [52,53], and lasts up to 3 hours post administration. In contrast to the three hour 200% enhanced sucralfate concentration observed using sucralfate gel, PPSBT at three hours has an 800% enhanced sucralfate concentration on normal mucosa and 2,400% enhanced concentration on inflamed mucosa. Given that the entirety of sucralfate’s clinical effect depends on the surface concentrations achieved following administration, PPSBT should have clinical outcomes commensurate to its augmented accumulation on the mucosal lining. Indeed, that seemed to be the case. In this study, three grams of PPSBT taken over 28 days achieved a positive response in 87.3% of participants compared to 71% of patients using sucralfate gel over 42 days.
Conclusion
This randomized double-blind placebo controlled trial on the efficacy and safety of pre-polymerized sucralfate barrier therapy found it to be safe and effective in patients with symptomatic NERD. There are no current management guidelines for NERD. The Vevey NERD Consensus Group, which excludes functional heartburn and reflux hypersensity from the NERD, recommend use of acidcontrolling therapies, PPI’s, H2RA and antacids where useful [54]. However, clearly the similarity of histomorphology, transmembrane resistance, and immunoreactions suggest that esophageal exposure to gastric refluxate likely has a decisive role in symptoms regardless of sub-type of NERD present. Thus, for all sub-types of NERD, physical exclusion of refluxate (or of dissolved irritants) is key. As a class, sucralfate biophysically prohibits access of irritants to the esophageal mucosa and PPSBT is a form of sucralfate capable of achieving enhanced concentrations in mucin without increasing its administered dose. Significant resolution of NERD symptoms by PPSBT reported here in this undifferentiated group of NERD patients statistically verifies its usefulness.
Acknowledgement
Contracted Trial Director: Professor AK Azad Khan delegated all logistical responsibilities.
Contracted Lead Coordinator: Dr. Mian Mashhud Ahmad was responsible for coordinating patient recruitment, blinding, management of endoscopies, assessment of patient diaries and scoring of patient outcomes.
Contributing Investigators: The following gastroenterologists were tasked with patient assessment, endoscopies, and test drug distribution - Dr. M.A. Masud, Dr. Swapan Chandra Dhar, Dr. Md. Habibur Rahman, Dr. Dewan Saifuddin Ahmad, Dr. Hafeza Aftab, Dr. Hasan Masud, Dr. Naima Haque, Dr. Anisur Rahman, Dr. Mahmud Hasan.
Medical Institutions: Department of Gastrointestinal & Liver Diseases, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh; Department of Gastroenterology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh; Department of Gastrointestinal, Hepatobiliary and Pancreatic Disorders (GHPD) and Research Division, Bangladesh Institute of Research & Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM), Dhaka, Bangladesh
Trial Concept, Methodology, Data curation, analysis, literature research, writing and editing manuscript: R. W. McCullough MD
Conflict of Interest
Author is employed by company who owns technology that prepolymerizes sucralfate. The company had no role in the execution of the study or interpretation of data.
References
- Huo X, Zhang X, Yu C, Zhang Q, Cheng E, et al. (2014) In oesophageal squamous cells exposed to acidic bile salt medium, omeprazole inhibits IL-8 expression through effects on nuclear factor kB and activator protein-1. Gut 63(7): 1042-1052.
- Yu Y, Ding X, Wang Q, Xie L, Hu W, et al. (2011) Alterations of mast cells in the esophageal mucosa of the patients with non-erosive reflux disease. Gastroenterol Res 4(2): 70-75.
- Kim JJ, Kim N, Choi YJ, Kim JS, Jung HC (2016) Increased TRPV1 and PAR2 mRNA expression levels are associated only with the esophageal reflux symptoms but not with extrasophageal reflux symptoms. Mediine (Baltimore) 95(32): e4387.
- Yoshida N, Kamada K, Tomatsuri N et al. (2011) Non-erosive reflux disease and neurogenic inflammation: Association of circulating Substance P and Nerve Growth Factor levels with heartburn symptoms. Gastroenterol 140(5).
- Park JM, Chi KC (2018) Antireflux surgery is equally beneficial in nonerosive and erosive gastroesophageal reflux disease. Ann Surg Treat Res 95(2): 94-99.
- Morris GP (1995) Binding of sucralfate to mucosal surface. In Sucralfate: from basic science to the bedside. Daniel Hollander, GNJ Tytgat (eds.), Plenum Press, New York.
- Cohen MM, Bowdler R, Gervais P, Morris GP, Wang HR (1989) Sucralfate protection of human gastric mucosa against acute ethanol injury. Gastroenterol 96: 292-298.
- Tasman-Jones C, Morrison G, Thomsen L, vanDerwee M (1989) Sucralfate interactions with gastric mucus. Am J Med 86(suppl6A): 5-9.
- Slomiany BL, Murty VL, Piotrowski J, Slomiany A (1989) Effect of antiulcer agents on the physiochemical properties of gastric mucus. Symp Soc Exp Biol 43: 179-191.
- Slomiany BL, Laszewicz W, Murty VL, Kosmala M, Slomiany A (1985) Effect of sucralfate on the viscosity and retardation of hydrogen ion diffusion by gastric mucus glycoprotein. Comp Biochem Physiol C 82(2): 311-314.
- Slomiany BL, Liu J, Slomiany A (1992) Modulation of gastric mucosal calcium channel activity by sucralfate. Biochem Int 28(6):1125-1134.
- Caspary WF (1995) Binding of bile salts by sucralfate. In Sucralfate: from basic science to the bedside. Daniel Hollander, GNJ Tytgat (eds.), Plenum Press, New York.
- Schweitzer EJ, Bass BL, Johnson LF, Harmon JW (1985) Sucralfate prevents experimental peptic esophagitis in rabbits. Gastroenterol 88: 611-619.
- Louw JA, Young GO, Winter TA, Marks IN (1995) Sucralfate and Helicobacter Pylori. In Sucralfate: from basic science to the bedside. Daniel Hollander, GNJ Tytgat (eds.), Plenum Press, New York.
- Slomiany BL, Piotrowski J, Slomiany A (1992) Effect of sucralfate on the degradation of human gastric mucus by helicobacter pylori protease and lipases. Am J Gastroenterol 87(5): 595-599.
- Vaira D, Corbelli C, Brunetti G, Menegatti M, Levorato M, et al. (1993) Gastric retention of sucralfate gel and suspension in upper gastrointerstinal diseases. Aliment Pharmacol Ther 7: 531-535.
- Rossi S, Bonferoni MC, Caramella C (1992) Rheological study of sucralfate humid gel: a contribution to comprehension of its stability properties. Eur J PHarm Biopharm 38: 78-81.
- Modlin IM, Hunt RH, Malfertheiner P, Moayyedi P, Quigley EM, et al. (2009) Diagnosis and management of non-erosive reflux disease- the Vevey NERD Consensus Group. Digestion 80(2): 74-88.
-
Ricky Wayne McCullough. Efficacy and Safety of Esolgafate, A Pre-Polymerized Cross-Linked Sucralfate Medical Device for NERD. A Randomized Double-Blind Placebo-Controlled Trial. Acad J Gastroenterol & Hepatol. 2(2): 2020. AJGH.MS.ID.000532.
-
Polymerized sucralfate, NERD, Barrier therapy, Sucralfate suspension, Gastroesophageal reflux disease, Reflux hypersensitivity syndrome, Heartburn, Esophageal epithelium, Headache, Diarrhea, Abdominal pain
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






