 Research Article
Research Article
Bowel Preparation for Flexible Sigmoidoscopy: Is a Phosphate Enema Sufficient?
Mahmoud AbdelDayem1*, Michael N Frempong1, Jennifer Waterman1, Melanie Fuller1 and PN Haray1,2
1Department of Colorectal Surgery Prince Charles Hospital, UK
2University of South Wales, UK
Mahmoud Abdeldayem, Department of Colorectal Surgery Prince Charles Hospital, UK.
Received Date: September 10, 2019; Published Date: September 17, 2019
Abstract
Aim: To explore the efficacy of phosphate enemas as bowel preparation for flexible sigmoidoscopy.
Methods: A retrospective study of patients underwent flexible sigmoidoscopy at District General hospital over a four-month period, from June to October 2017.
Results: A total of 428 patients underwent flexible sigmoidoscopy during the study period, 37 patients excluded as they had incomplete procedure due to reasons other than bowel preparation (16 dues to patient discomfort and 21 due to technical / clinical judgement reasons) and 391 patients included in our study. The bowel preparation used was as follows; 239 patients (61.1%) had Moviprep®, 130 (33.2%) had phosphate enema, 11 (2.8%) had Picolux®, 2 (0.5%) had Clean Prep® and 9 patients had no Preparation prior to the procedure
Originality Statement
This subject always a debate, Many centres moving to Oral bowel preparation which is more effective but carry a risk on elderly patients with co-morbidities, Enema is 80% efficient and Should be standardized for Bowel preparation for flexible sigmoidoscopy with the Oral Preparation kept for ones failed with enema.
Discussion and Conclusion
A single phosphate enema, administered one hour prior to procedure, was deemed effective in the vast majority of patients. The authors conclude that this should be established as the first line of bowel preparation for flexible sigmoidoscopy, reserving the use of oral preparation for those patients where adequate visualization could not be achieved by enema alone.
Background
Bowel preparation is crucial to undertake flexible sigmoidoscopy, as poor views of the bowel mucosa can result in an inadequate examination and the need for further investigations. Endoscopists develop personal preferences and patient tolerance and side effects also have an impact on the choice of the bowel preparation used. Endoscopy units also have different protocols which can be administered orally or rectally.
Aim
Our aim was to explore the efficacy of phosphate enemas as bowel preparation for flexible sigmoidoscopy.
Methods
We undertook a retrospective study of patients who underwent flexible sigmoidoscopy at our hospital over a four-month period, from June to October 2017. Data was extracted from online endoscopy records with specific emphasis on patient demographics, details of the endoscopist, the indication, the bowel preparation used, the adequacy of the preparation, the site of insertion of the endoscope reached by the endoscopist compared to the intended site and rescoping decision in cases with inadequate preparation.
Endoscopists gave subjective ratings of the quality of the bowel preparation as excellent, good, adequate and inadequate after completion of each procedure. The ratings were determined on the basis of the percentage of bowel mucosa that was visible to the endoscopist. We use a modified the rating scale of bowel preparation based on the more commonly used Aronchick and Ottawa Scales.
Results
A total of 428 patients underwent flexible sigmoidoscopy during the study period, 37 patients excluded as they had incomplete procedure due to reasons other than bowel preparation (16 due to patient discomfort and 21 due to technical/clinical judgement reasons) and 391 patients included in our study. The bowel preparation used was as follows; 239 patients (61.1%) had Moviprep® (Macrogel 3350 with Anhydrous sodium sulfate, ascorbic acid, potassium chloride, sodium ascorbate and sodium chloride), 130 (33.2%) had phosphate enema, 11 (2.8%) had Picolax® (Magnesium citrate with sodium picosulfate), 2 (0.5%) had clean Prep® (Macrogel 3350 with anhydrous sodium sulfate, potassium chloride, sodium bicarbonate and sodium chloride) and 9 patients had no Prep prior to the procedure (Figure 1).
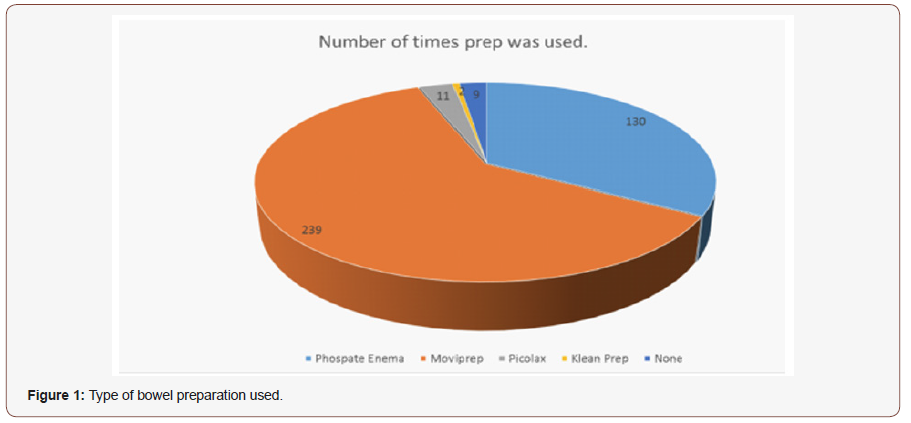
Overall therefore, 64.4% of patients had oral preparation and 33.2% had enema preparation. 354 (90.5%) were recorded as complete procedures as the intended site in the colon was reached and the preparation was either excellent, good or adequate; While the remaining 37(9.55%) had incomplete procedures due to poor bowel preparation. A procedure was deemed inadequate if either there was a poor result from the bowel preparation and/or the intended site of the investigation was not reached. Out of the 130 patients who received Phosphate Enemas as bowel preparation, 104 (80%) had a good result allowing for complete procedure. 26 (20 %) were noted to have inadequate bowel preparation and hence incomplete procedure.
239 patients had Moviprep® as their bowel preparation for the flexible sigmoidoscopy, of which only 8 (3.34 %) patients had incomplete procedures. 11 patients had Picolax of which 90 % had complete procedures. The two patients receiving clean Prep both had complete procedures (100%) (Figure 2).
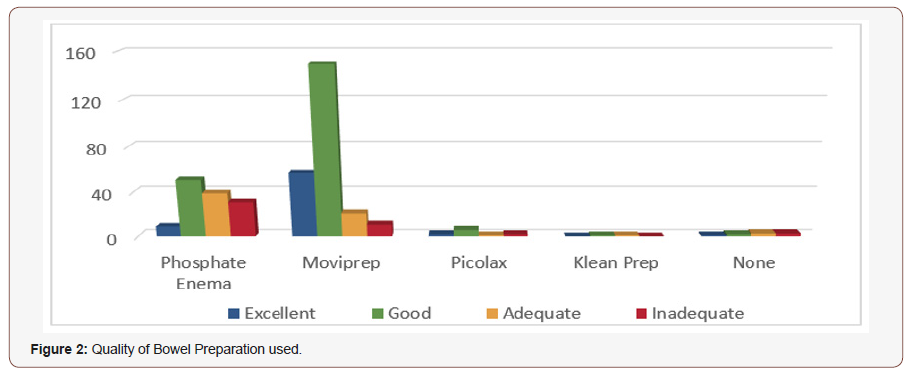
It is observed in our practice that Adverse effects including nausea, vomiting, abdominal pain and discomfort were seen more often in the patients who received oral bowel preparations (some of whom were even admitted as emergencies), compared with those who received enemas. However, for the purposes of this study, we have not collected or analysed this data. There was no statistical difference between the age groups of patients having enema versus oral preparations. (median 72.6 years and 64.1 years respectively).
Discussion
Hookey L et al. [1] in their randomized trial of 305 patients, compared a single dose of low volume oral laxative (picosulfate with magnesium citrate-P /MC) administered the night before on its own, in combination with an enema and an enema on its own. They concluded that the P/MC regimens were not superior to the enema on its own in achieving colon cleansing for sigmoidoscopy [3]. They also noted that both oral P/MC (with or without enema) regimens were associated with higher incidence of nausea, abdominal pain, bloating, and interrupted sleep than enema alone (P<0.05) [1]. This is in line with our results, that phosphate enema alone is a sufficient and effective bowel preparation for a flexible sigmoidoscopy in the majority of patients. A detailed study by Atkin W et al. [4] looked at oral picolax® versus self-administered phosphate enema as bowel preparation for flexible sigmoidoscopy [5]. The study involved 1442 patients and found compliance with the enema was higher than with picolax®. They also reported that enemas yielded a better quality of preparation, achieved a higher proportion of complete procedures (up to the descending colon) as well as a shorter mean duration of the procedure. They also reported a higher incidence of adverse effects with picolax® compared with the enema and concluded that an enema was a more acceptable and effective method of preparing the distal bowel for flexible sigmoidoscopy than Picolax®.
Drew PJ et al. [5] randomized one hundred and two consecutive patients to a self-administered phosphate enema versus Picolax® as preparation for flexible sigmoidoscopy. Self-administered enemas provided a significantly superior bowel preparation with 93% being judged adequate or better, as opposed to 74% in the Picolax® group. In addition, enemas were associated with significantly fewer adverse symptoms (20% vs 52%). Another large prospective randomized single-blind study with 3015 patients by Gidwani et al [2] compared 3 regimes for preparation: group 1: one phosphate enema two hours pre-procedure; group 2: two phosphate enemas, one on the evening prior to sigmoidoscopy and one two hours pre-procedure; group 3: lactulose 30 ml orally 48 and 24 hours prior to sigmoidoscopy, plus a single Fleet enema two hours pre-procedure. Their conclusion was that a single phosphate enema was as effective as the other two regimes. These results are similar to our experience, though in our series, the phosphate enema is administered one hour prior to the procedure rather than two hours, as in the above study.
Conflicting views have been described by Bini, et al. [6], Sharma et al. [7], who have reported a superior quality of preparation with the use of oral regimens the evening before an elective sigmoidoscopy compared with the typical “enema-only”-based regimens. However, it must be noted that the study by Sharma et al. [7] included a cohort of 70 patients only. The cost of the bowel preparation in our study was calculated based on the cost of the bowel preparation in addition to the cost of secure postal delivery service used to deliver the oral bowel preparations to the patients (delivery cost 3.95£). The average cost of the oral preparations is Moviprep® is 10.36£, The cost of the Klean prep® is 10.48£. In Comparison, the phosphate enema costs only £3.98 with no delivery cost (as it is administered on attendance at the endoscopy unit).
Although in our series a phosphate enema achieved a lower proportion of adequate bowel preparation compared with oral preparations, it was still adequate in 80% of cases. Hence, using a phosphate enema routinely in all cases would have avoided the need for oral preparation in over three quarters of patients. In our unit during the year 2017 we have performed 2152 flexible sigmoidoscopes and if the enema is effective in 80% of them (1721 patients) , based on the costs above, it would result in an annual saving of £17777.93.Thus, there could be potential benefits both from the perspectives of patient safety as well as cost effectiveness.
Conclusion
A single phosphate enema, administered one hour prior to procedure, was deemed effective in the vast majority of patients. The authors conclude that this should be established as the first line of bowel preparation for patients undergoing flexible sigmoidoscopy, reserving the use of oral preparation for those few patients where adequate visualization could not be achieved by enema alone.
Acknowledgment
Mahmoud Abdeldayem “MD, FRCS England” - Specialty Doctor of Genera and Colorectal Surgery: Main Author, Data revision, Paper writing and took part in Data Collection.
Michael Frempong “MBBS” - Foundation Year 1 Doctor and Jennifer Waterman “MRCS England” - Specialty Trainee of Surgery Year 1: Data collection and Literature search.
Melanie Fuller - Specialist Nurse Endoscopist: Data Collection and review.
P.N. Haray - Consultant Colorectal Surgeon: Supervision, Paper review and correction.
Conflict of Interest
No Conflict of Interest.
References
- Singh K, Wang ML, Ofori E, Widmann W, Alemi A, et al. (2012) Gallstone abscess as a result of dropped gallstones during laparoscopic cholecystectomy. Int J Surg Case Rep 3(12): 611-613.
- Khalid M, Rashid M (2009) Gallstone abscess: a delayed complication of spilled gallstone after laparoscopic cholecystectomy. Emerg Radiol 16(3): 227-229.
- Ragozzino A, Puglia M, Romano F, Imbriaco M (2016) Intra-Hepatic Spillage of Gallstones as a Late Complication of Laparoscopic Cholecystectomy: MR Imaging Findings. Polish J Radiol 81: 322-324.
- Rice DC, Memon MA, Jamison RL, Agnessi T, Ilstrup D, et al. (1997) Longterm consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1(1): 85-91.
- Zehetner J, Shamiyeh A, Wayand W (2007) Lost gallstones in laparoscopic cholecystectomy: all possible complications. Am J Surg 193(1): 73-78.
- Schäfer M, Suter C, Klaiber C, Wehrli H, Frei E, et al. (1998) Spilled gallstones after laparoscopic cholecystectomy. A relevant problem? A retrospective analysis of 10,174 laparoscopic cholecystectomies. Surg Endosc 12(4): 305-309.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL (2004) Spilled gall stones during laparoscopic cholecystectomy: A review of the literature. Postgrad Med J 80(940): 77-79.
- Brockmann JG, Kocher T, Senninger NJ, Schürmann GM (2002) Complications due to gallstones lost during laparoscopic cholecystectomy. Surg Endosc Other Interv Tech 16(8): 1226-1232.
- Johnston S, O Malley K, McEntee G, Grace P, Smyth E, et al. (1994) The need to retrieve the dropped stone during laparoscopic cholecystectomy. Am J Surg 167(6): 608-610.
- Horton M, Florence MG (1998) Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 175(5): 375-379.
-
Mahmoud AbdelDayem, Michael N Frempong, Jennifer Waterman, Melanie Fuller, PN Haray. Bowel Preparation for Flexible Sigmoidoscopy: Is a Phosphate Enema Sufficient?. Acad J Gastroenterol & Hepatol. 1(3): 2019. AJGH.MS.ID.000513.
-
Sigmoidoscopy, Demographics, Endoscopist, Colorectal surgery, Phosphate enema, Nausea, Abdominal pain, Bloating
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






