 Case Report
Case Report
New Onset Crohn’s Disease with Ixekizumab Initiation
Jena Velji Ibrahim1,2* and Kalpit Devani1,2,3
1Department of Internal Medicine, Prisma Health Upstate, USA
2University of South Carolina School of Medicine, USA
3Prisma Health Gastroenterology and Liver Center, USA
J Velji Ibrahim, Department of Internal Medicine, Prisma Health-Upstate, University of South Carolina School of Medicine, USA
Received Date:February 05, 2025; Published Date:February 19, 2025
Abstract
Ixekizumab, a monoclonal antibody targeting interleukin-17, is a potent therapy for plaque psoriasis and spondyloarthropathies. Although clinical trials have reported rare cases of inflammatory bowel disease (IBD), biologics may induce or exacerbate IBD. We present a case of a male in his mid-60s with psoriasis and psoriatic arthritis who developed new onset ixekizumab-associated colitis, complicated by pulmonary embolism and hemorrhagic shock precipitated by gastrointestinal bleeding. Colonic pathology demonstrated features of Crohn’s disease. The patient remained disease-free following ixekizumab cessation. This case demonstrates an association between ixekizumab and IBD, calling on clinicians to be mindful of potential risks before prescribing anti-IL-17 agents.
Abbreviations:Interleukin-17 inhibitors, ixekizumab, inflammatory bowel disease
Background
Interleukin-17 (IL-17), a proinflammatory cytokine, has emerged as a key target in biologic therapy of various autoimmune diseases. Three IL-17 inhibitors, ixekizumab, secukinumab, and brodalumab are beneficial in the treatment of plaque psoriasis and spondyloarthropathies [1]. Despite only infrequent reports of inflammatory bowel disease (IBD) in clinical trials, there have been increasing reports of new-onset IBD in association with IL- 17 agents [2-5]. Here, we report a case of a patient with psoriasis and psoriatic arthritis treated with ixekizumab who presented with chronic diarrhea, complicated by pulmonary embolism and hemorrhagic shock. He was ultimately diagnosed with new-onset Crohn’s disease.
Case Presentation
A male in his mid-60s with a 7-year history of psoriasis and psoriatic arthritis presented to the emergency department with five months of large-volume watery diarrhea, dyspnea, and multiple falls. He described having eight to ten large-volume, watery bowel movements daily. During the five months, he reported a substantial weight loss of 40 pounds. He denied recent travel, sick contacts, or a history of IBD. Notably, the patient had initiated ixekizumab therapy approximately one month before symptom onset.
He has a history of hypothyroidism, hypertension, and insomnia. His home medications included levothyroxine 75 mcg/day, losartan 100 mg/day, metoprolol 100 mg twice/day, and amitriptyline 10 mg/night.
Previous treatments for psoriasis and psoriatic arthritis include methotrexate 15 mg/weekly which was stopped due to intolerance and etanercept 50 mg/weekly. However, because the patient had a flare despite etanercept treatment, he was switched to ixekizumab 80 mg/weekly after induction. After only one month of ixekizumab, the patient developed a large volume of watery diarrhea.
On initial evaluation, the patient was tachycardic to 122 beats per minute. Laboratory tests revealed elevated inflammatory markers, with a C-reactive protein (CRP) of 64 mg/L and fecal calprotectin of 9867 μg/g. The infectious workup was negative. Computed tomography (CT) of the abdomen and pelvis demonstrated bowel wall thickening suggestive of colitis in the descending, transverse, and sigmoid colon with involvement of the splenic flexure (Figure 1A). However, before the patient’s colonoscopy, he developed chest pain in addition to persistent tachycardia. The workup unveiled a left popliteal vein DVT and bilateral pulmonary thromboemboli for which he was started on enoxaparin 90 mg/twice daily. Interestingly, the patient’s diarrhea improved with dicyclomine 10 mg/four times daily as needed and loperamide 2 mg/three times daily as needed, allowing for the deferral of colonoscopy to an outpatient setting, and the patient was discharged.
Unfortunately, two weeks later, the patient returned to the ED with pleuritic chest pain, dyspnea, and diarrhea. He discontinued enoxaparin therapy as he didn’t understand the necessity for its continuation. On evaluation, he was tachycardic to 140 beats per minute with a temperature of 100.8°F. Further investigation revealed a notable increase in CRP to 197 mg/L. The infectious workup was again negative. A CT pulmonary angiogram illustrated worsened pulmonary embolus thrombus burden, including a new thrombus occluding the entire right lower lobe pulmonary arteries and a new embolus in the left lower lobe, for which the patient was heparinized. A repeat CT scan of the abdomen and pelvis was significant for colitis, consistent with findings from the previous scan. Due to his persistent diarrhea, a flexible sigmoidoscopy was performed, showing patchy ulceration, granulation tissue, and reactive epithelial changes in the colon. Biopsies were not significant for histopathologic changes.
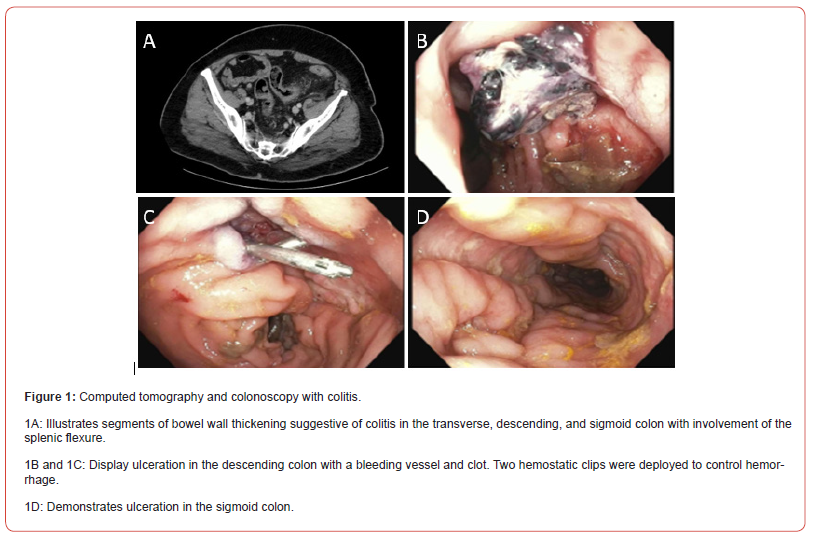
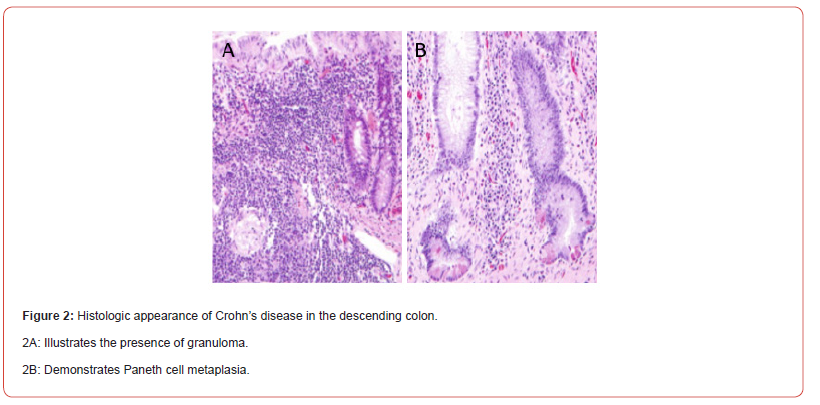
The patient’s clinical course was further complicated by hemorrhagic shock, precipitated by hematochezia with a hemoglobin drop from 11 to 5.8 g/dL. After stabilization in the intensive care unit, an inferior vena cava filter was placed and the patient underwent colonoscopy revealing ulceration in the transverse, descending, and sigmoid colon (Figures 1B-1D). Additionally, a visible vessel below a clot in the descending colon necessitated hemostatic intervention with two hemostatic clips (Figures 1B & 1C). Biopsies were consistent with Paneth cell metaplasia and a small granuloma, concerning for Crohn’s disease (Figures 2A & 2B). In light of these findings, ixekizumab therapy was discontinued.
Based on the patient’s colonoscopy findings, ixekizumab was felt to be suspicious for this adverse event. The patient was started on budesonide 9 mg at hospital discharge. At his gastroenterology clinic appointment one month later, he reported continued bowel movements every two hours. He was initiated on infliximab and azathioprine. After induction, he was continued on 500 mg every 8 weeks and azathioprine 50 mg/daily. One month later, he was symptom-free and had a CRP of 5 mg/L. The patient has remained asymptomatic since ixekizumab was discontinued. The adverse event was reported to the Food and Drug Administration.
Discussion
Ixekizumab is one of three IL-17 inhibitors approved for the treatment of psoriasis and spondyloarthropathies. In the initial efficacy trials for ixekizumab, new cases of IBD were uncommon (<1%) [2]. Similarly, trials evaluating other anti-IL-17 agents also reported only rare instances of IBD [6]. Despite increasing reports of inflammatory bowel pathologies in patients treated with an IL-17 inhibitor, recent studies have not indicated a significantly increased risk of developing IBD with exposure to IL-17 antagonists [4,5,7,8]. Furthermore, a recent meta- analysis of 17 randomized trials and over 18,000 patients with moderate to severe psoriasis, with up to five years of exposure to ixekizumab, resulted in only 31 cases of confirmed IBD [3]. While these studies emphasize that de novo IBD due to IL-17 inhibition is rare, the collection of IBD history in several studies was based on voluntary reporting and participants were not systematically asked about personal and family history of IBD; raising concern that the true incidence of IBD may be underreported [9].
Psoriatic diseases have been associated with IBD and other gastrointestinal pathologies including irritable bowel syndrome and celiac disease [10]. However, the underlying pathophysiology that links psoriasis and IBD is not well understood. IBD pathogenesis involves a complex network of cytokines associated with intestinal inflammation [11]. In particular, interactions between IL-12, IL- 23, IL-17, and TNF-alpha play important roles in gut defense mechanisms and the regulation of tissue inflammation [6,12]. While ixekizumab binds IL-17, blocking its action and thereby reducing symptoms of psoriasis and spondyloarthropathies, the role of IL-17 in IBD is unclear. Previous animal studies have yielded conflicting findings regarding the effectiveness of IL-17 inhibitors in treating intestinal inflammation [13]. Furthermore, studies of secukinumab, another IL-17 inhibitor, found no benefit in the treatment of Crohn’s disease in some patients, with a subset experiencing a worsening of IBD symptoms after treatment [14]. Given the intricacy of innate immunity and cytokine pleiotropy in different tissues, it is not surprising that the inhibition of one cytokine may have opposing effects on two related conditions.
Our patient had no previous personal or family history of IBD. We conclude that he developed de novo Crohn’s disease as a result of using ixekizumab. Although the precise mechanisms of action in the gut mucosa remain unclear, our case raises concerns that IL- 17 inhibition may ameliorate one inflammatory condition while exacerbating the other. We recommend that future cases involving such associations be reported to enhance post-market surveillance. Furthermore, we suggest that, before initiating treatment with anti-IL-17 agents, patients undergo a comprehensive assessment of their personal and family history for IBD and are counseled on this association.
Author Contributions
J Velji-Ibrahim wrote and revised the manuscript. K Devani revised the manuscript and is the article guarantor.
Financial Disclosure
None to report
Declaration of Competing Interest
The authors have no other relationships, conditions, or circumstances that present a potential conflict of interest. Informed consent was obtained for this case report.
References
- Onishi RM, Gaffen SL (2010) Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 129: 311-321.
- Gordon KB, Blauvelt A, Papp KA, et al. (2016) Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 375(4): 345-356.
- Griffiths CEM, Gooderham M, Colombel JF, et al. (2022) Safety of ixekizumab in adult patients with moderate-to-severe psoriasis: Data from 17 clinical trials with over 18,000 patient-years of exposure. Dermatol Ther (Heidelb) 12(6):1431-1446.
- Nazarian A, Grin A, Wijeratne DT (2020) Ixekizumab associated new-onset inflammatory bowel disease. ACG Case Rep J 7(2): e00316.
- Mu X, Fardy J, Reid S, et al. (2021) Severe drug-associated colitis with Crohn's features in setting of ixekizumab therapy for chronic plaque psoriasis. BMC Gastroenterol 21(1): 361.
- Moschen AR, Tilg H, Raine T (2019) IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 16(3): 185-196.
- Yamada A, Wang J, Komaki Y, et al. (2019) Systematic review with meta-analysis: risk of new onset IBD with the use of anti-interleukin-17 agents. Aliment Pharmacol Ther 50(4): 373-385.
- Wright S, Alloo A, Strunk A, et al. (2020) Real-world risk of new-onset inflammatory bowel disease among patients with psoriasis exposed to interleukin 17 inhibitors. J Am Acad Dermatol 83(2): 382-387.
- Reich K, Leonardi C, Langley RG, et al. (2017) Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: A presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol 76(3): 441-448.e2.
- Zohar A, Cohen AD, Bitterman H, et al. (2016) Gastrointestinal comorbidities in patients with psoriatic arthritis. Clin Rheumatol 35(11): 2679-2684.
- Friedrich M, Pohin M, Powrie F (2019) Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50(4): 992-1006.
- Leppkes M, Neurath MF (2020) Cytokines in inflammatory bowel diseases-update 2020. Pharmacol Res 158: 104835.
- Li J, Casanova JL, Puel A (2018) Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol 11(3): 581-589.
- Hueber W, Sands BE, Lewitzky S, et al. (2012) Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double- blind placebo-controlled trial. Gut 61(12): 1693-1700.
-
Jena Velji Ibrahim* and Kalpit Devani. New Onset Crohn’s Disease with Ixekizumab Initiation. Acad J Gastroenterol & Hepatol. 4(2): 2025. AJGH.MS.ID.000582.
-
Diarrhea; Hemorrhagic shock; Ixekizumab; Dyspnea; gastrointestinal pathologies; irritable bowel syndrome; celiac disease
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Track Your Article
Refer a Friend
Advertise With Us
Feedback
 a Creative Commons Attribution 4.0 International License. Based on a work at www.irispublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.irispublishers.com.
Best viewed in





