 Research Article
Research Article
Effect of Preadmission Proton Pump Inhibitor (PPI) on the clinical outcome of Covid-19 Hospitalised Patients during the Pandemic
Tasneem Elkanzi, Eugenia Ennin, YKS Viswanath*, Awwab Shahid, Mustafa Omar, Rayan Mahmoud, Marwa Elhossary, Tan Ker
Professor of Surgery, Consultant Surgeon (Upper GI and Laparoscopic), South Tees Hospitals NHS Foundation Trust, James Cook University Hospital, UK
YKS Viswanath, Professor of Surgery, Consultant Surgeon (Upper GI and Laparoscopic), South Tees Hospitals NHS Foundation Trust, James Cook University Hospital, UK
Received Date:November 10, 2023; Published Date:November 17, 2023
Abstract
Methodology: Prospectively captured observational data was analyzed to include patients (>18 yr.) at the hospital with COVID-19 infection. PPI data was derived from hospital and primary care records, and the study period is between February 2020 and February 2021. Clinical outcomes of COVID-19 patients who were on proton pump inhibitors preadmission were compared with those of COVID-19 patients who were not on proton pump inhibitors simultaneously. The study’s primary endpoint was 60-day mortality, intensive care unit admission, high dependency unit admission, and the development of COVID-19 complications. Additional endpoints included the length of critical care admission.
Study type: Observational Cohort Study.
Results: 309 patients were evaluated in this study; 159 were on proton pump inhibitors, and 150 were not on proton pump inhibitors at index admission. The mean length of stay was 9.8 in the PPI group and 12.1 in the non-PPI group. A slightly increased mortality rate of 22.6% in the PPI group compared with 19.3 % in the non-PPI group. Intensive Care Unit (ITU) and High Dependency Unit (HDU) admissions were higher in the PPI group (56.6%,30.8% respectively) than in the non-PPI group (45.3%,20.7%). Complications were more common in the PPI group74.8% had pulmonary complications, and 3.1% had thromboembolic complications. In the non-PPI group, 54% had pulmonary complications, which was over 20 % less than in the PPI group, 6% had thromboembolic complications, 1.93 times more than the PPI group.
Conclusion: In Our study, PPI usage at index admission succeeded in showing worsening of outcomes in Covid 19 hospitalised patients, similar to recently published papers. This proposed causation needs further evaluation via well-conducted prospective studies.
Introduction
COVID-19 has proved to be a severe disease since the end of 2019; according to WHO, over 600 million people have contracted this infection [1]. Most people who contracted this disease showed no symptoms. Initially, the mean hospitalisation period for symptomatic patients was 16 days, ranging between 3 to 45 days [2]. A significant complication of COVID-19 was found to be respiratory hypoxaemia, which accounted for up to 75% of hospital admissions secondary to pneumonia, along with acute respiratory disease (ARDS). While prodromal systems, such as fever, cough and shortness of breath, were seen in most symptomatic Covid patients, many system involvements were also observed. For instance, 19.7% of patients were found to have a cardiac injury [3]. Similarly, many systems involvements have been observed in SARS patients: 36.4% showed neurological manifestations of their disease [4], 19% had liver abnormalities [5], and 27-35.35% had venous and arterial thromboembolic events [6].
This research focuses on the effects of proton pump inhibitors (PPI) on SARs-COVID-19. We investigated whether PPI use at preadmission affected clinical outcomes among COVID-19 hospitalised patients. PPIs are effectively used to treat acid-related diseases, most commonly gastro-oesophageal reflux disease and peptic ulcer disease. They irreversibly bind the hydrogen-potassium ATPase gastric proton pump of parietal cells, thus resulting in a raised stomach pH [7]. The consequent hypochlorhydria that ensues has been demonstrated to predispose individuals to an increased risk of viral infection, namely rotaviruses, noroviruses and coronaviruses. This would then explain the possibility that patients taking PPIs will have an internal environment favouring the survival of COVID-19 [8] and allows an adequate amount of time to enable the virus to infiltrate epithelial cells of the gastrointestinal tract and as mentioned, enhance the risk of developing covid-19 infection [9]. PPIs are commonly prescribed medications to UK patients; they impact several organs in the body, potentially resulting in renal disease, liver disease, fracture risks and electrolyte disturbances [10]. The effect of PPI on COVID-19 has been previously researched and analysed to show a marginal increase in the risk of contracting COVID-19 [11]. PPI use in COVID-19-positive patients was found to have a 9 in 10 greater probability of having severe consequences of Covid [12]. In addition, many medications have already been researched to influence COVID-19 outcomes; therefore, analysing the relationship between COVID-19 and PPIs is essential, considering their availability and frequent use.
The history of viral entities reveals plenty; Sars-Cov-1, for instance, appears to have reduced activity in an acidic environment [13]. Patients on acid-suppression treatments have previously been found to have Influenza RNA in their gastric tissue, which implies that a pH skewed further towards the neutral/basic range could implicate patients to gastric influenza infection [14]. Infections of viral aetiology, such as several community-acquired infections, are more prevalent in patients consuming anti-acid treatments [15]. Furthermore, using norovirus as an example, studies have shown that patients with acid-suppressive therapy are at an increased risk of contracting the infection compared to counterparts not taking said therapies [16]. As a result, the regular undertaking of treatments such as proton pump inhibitors has highlighted an increased risk of contracting viral enteric infections [17]. Shifting the focus to the coronaviruses group of infections, experiments were done on mice where the viral load of MERS-CoV was measured from the small intestines of the mice having been injected with the virus and compared with mice who also received the virus injection but had been treated with pantoprazole before injection. The results showed that the mice receiving pantoprazole also had higher viral loads than those not treated [18].
An American-based health survey done on a national scale illustrated a high probability of producing a positive COVID-19 test result correlated with the use of proton pump inhibitor(s). This particular study also outlined some of the pathophysiological mechanisms COVID-19 imposes, namely the fact that the virus disperses into saliva and transits to the stomach, resulting in gastrointestinal symptoms. To add to this further, the study also showed a dose-dependent relationship whereby individuals taking higher doses of proton pump inhibitors portrayed a higher probability of obtaining a positive COVID-19 test result when compared with individuals taking a lower dose [19]. Exciting data was gathered from another American retrospective cohort study done between March and April of 2020 at a tertiary centre in New York and included patients aged 18 years and over, which found that of the sampled patients, 15.6% of those were ultimately hospitalised with covid-19 were routinely consuming proton pump inhibitors t before infection and hospitalisation. To further potentially implicate the role of PPIs, there was a more than double effect on mortality when comparing patients taking PPIs vs those not taking PPIs (34.8% vs 16.2%). Unfortunately, a drawback of this particular study was that the researchers could not gather data on the type of PPI used or its dose and duration [8].
A Korean-based, more extensive retrospective cohort study analysed results from a national health insurance-based data pool and involved data collection of 132,316 citizens aged 18 years and over between January and May of 2020 and concluded that active utilisation of proton pump inhibitor treatment demonstrated an elevated chance of adverse severe clinical affectation of covid-19, however, did not imply a high prospect of being susceptible to the covid infection itself. The ultimate finding from this study was that PPI use, regardless of whether it was current active use or just prior use, had no bearing on the risk of contracting COVID-19 infection. However, individuals taking PPIs at the time of the study had a propensity to more adverse outcomes if infected with COVID-19 [12]. Similar results were found in another smaller retrospective cohort study done on confirmed cases of COVID-19 infection and were done to establish a causal link between PPI usage and resulting secondary infection in patients hospitalised due to COVID-19 infection. This study delved into pathophysiology and pharmacokinetics. Proton pump inhibitors, by default, impair the production of gastric acid, and this causes a bacterial build-up in the gastrointestinal tract. A pneumonic process occurs when aspiration occurs and leads to the colonisation of bacteria in the lung. The data gathered showed that just under 50% of individuals on PPI therapy developed a secondary infection compared to a much lower 20% in individuals not on PPI therapy. This data was inevitably skewed due to external risk factors such as comorbid statuses; however, even following adjustment for these factors, the difference in secondary infection development between taking PPI vs not taking PPI was still found to be statistically significant. To add fuel to the fire, this study also evidenced a heightened probability of developing ARDS when taking PPI therapy compared with not taking PPI therapy, and as such, these individuals conveyed a statistically significant raised index mortality [20].
Specific treatments, namely lysosomotrophic agents such as PPIs, Azithromycin, Indomethacin, NSAIDs and fluoxetine, have a natural ability to conduct antiviral action by way of affecting the mechanism of endolysosomal transit by mediating the pH. These substances can diffuse across endosomal membranes and may provide positive clinical and therapeutic results in the treatment of patients suffering from COVID-19 infection, and as such, PPIs may be helpful in not just treatment of COVID-19 but in the prophylaxis of it also [21,22]. Alternatively, some data highlights the potential benefits of proton pump inhibitor use in COVID-19-infected patients.
Research done in Germany focused on omeprazole evidenced that this PPI caused interference with the viral form of COVID-19 at sub-therapeutic concentrations in plasma. In addition, at therapeutic concentrations of omeprazole, the drug elevated the anti-covid-19 properties of aprotinin, which is a protease inhibitor, nearly 3-fold, as well as Remdesivir, a covid nucleotide analogue RNA polymerase inhibitor by 10-fold. This opens the door to the future management of COVID-19-afflicted patients as it suggests that there is scope for a potential combination treatment utilising the effects of omeprazole along with either aprotinin or remdesivir [23,24]. A Chinese-based retrospective study focused on patients who required admission to the Shanghai Public Health Centre concluded that they found no overall effect of PPI regimens on adults who were hospitalised with COVID-19 infection, both in evidencing a shorter or more prolonged duration of illness [25].
Methodology
Prospectively captured observational data was analysed to include patients aged 18 years and above with covid 19 infection at the James Cook University Hospital, Middlesbrough, United Kingdom, between February 2020 and February 2021. PPI data were derived from the hospital and Primary care records. Clinical outcomes of COVID-19 patients on PPI preadmission were compared with those not on PPI at the same time using p-value, standard deviation and Mann-Whitney test. Among hospitalised COVID-19-positive patients(n=309), 60-day mortality, intensive care unit admission, high dependency unit admission, development of COVID-19 complication and length of critical care admission among the PPI exposed group were compared with the non-PPI group.
Results
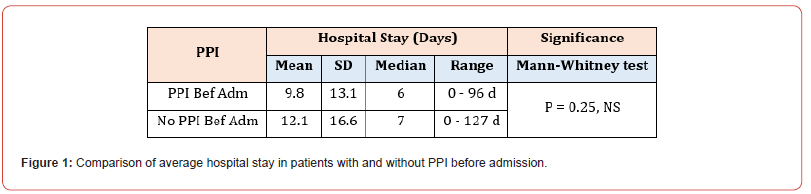
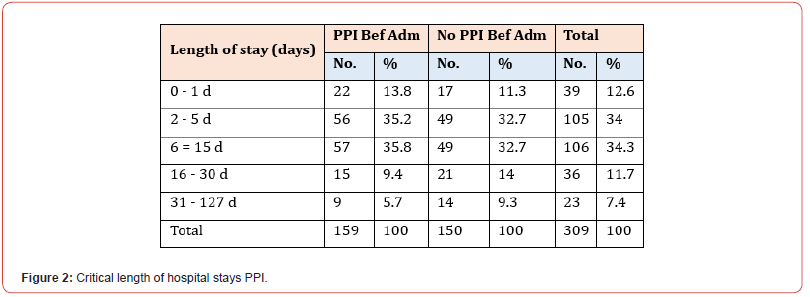
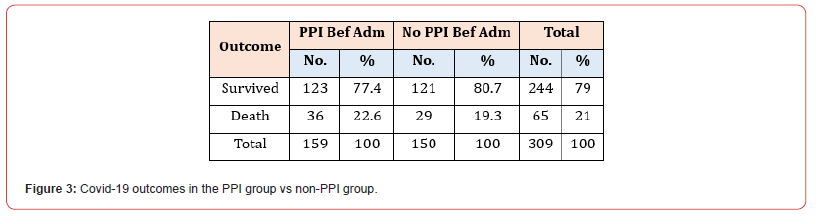
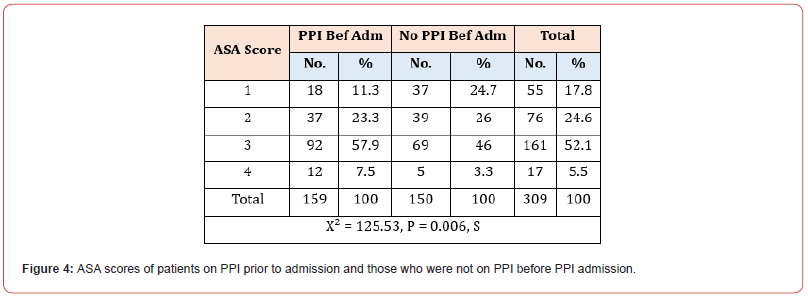
Among the 309 patients in our study, 159 were on PPI at index admission, while the remaining 150 had no significant history of PPI administration. The mean number of hospital days spent in the hospital was 9.8 (SD 13.1 days) for patients on PPI at index admission and 12.1 (SD 16.6 days) for patients not on PPI before the index admission. Moreover, the median number of days as an inpatient for patients on PPI before admission was six days vs seven days for patients who were not on PPI before admission. Mann- Whitney U test was used to differentiate between two independent groups when our dependent variable is ordinal (Figure 1). Most patients in both sub-groups had a hospital stay between 6-15 days, 35.8% in the PPI group and 32.7% in the non-PPI group (Figure 2). Concerning outcomes of COVID-19 in both subgroups, the mortality rate reached 22.6% in the PPI group vs 19.3% in the non-PPI group; however, this figure was statistically insignificant (X2=0.51) (Figure 3). In addition, COVID-19 patients who were on the PPI pre-admission index, admitted to HDU (30.8%), ICU (56.6%), vs Patients not on PPI before admission, admitted to HDU (20.7%), ICU (45.3%) The patients selected in this study had their ASA score assessed, and these results were compared in both sub-groups, i.e., patients on PPI before admission vs those not on PPI prior to admission. 161 (52.1%) patients in this study were found to have an ASA score of 3, while 55 (17.8%), 76 (24.6%), and 17 (5.5%) patients had an ASA score of 1,2 and 4 respectively. ASA score was highest in patients in the PPI group at 57.9% vs 46% in the non-PPI group. These figures were statistically significant, with a p-value of 0.006.
Data were analysed for both subgroups to identify the most common complications observed in our hospitalised COVID-19 patients. The primary consequence of SARS-COV was found to be respiratory in nature, which was seen in 119 patients (74.8%). This figure was lower in the non-PPI groups, with only 81 patients (54%). Other complications not seen as frequently were thromboembolic events, comprising 3.1% of patients in the PPI group and 6% of patients in the non-PPI group. Cerebral, cardiac, renal, endocrine, gastrointestinal and genitourinary results were also observed in the sample size, making up for 0.6%, 1.9%,1.3%,0%, 0.6%, and 1.3% in the PPI group, respectively, and 2.7%, 0.7%, 0.7%, 1.3%, 0.7% and 0%, respectively in the non-PPI group (Figure 4). Regarding the respiratory system, many of the outcomes seen were of covid pneumonitis; 113 (71.1%) patients in the PPI group were diagnosed with this, while a higher number of patients, accounting for 124 (82.7%) participants were found to have it in the non-PPI group. The remaining respiratory complications noted were Pleural effusion (0.6%), pneumothorax (1.9%), ventilatorassociated pneumonia (0.6%), hospital-acquired pneumonia (0.6%), cryptogenic organising pneumonia (0.6%) and pulmonary oedema (1.9%), in the PPI group. However, in the non-PPI group, we observed patients with ARDS (2.7%), pleural effusion (2%), pneumomediastinum (2%), Ventilator-associated pneumonia (2%), pneumothorax (1.3%), and hospital-acquired pneumonia (0.7%). The remaining complications seen in the non-PPI subgroup were as follows: AKI (3.3%), intracranial bleed (0.7%), Guillian Barre syndrome (0.7%), cardiac arrest (1.3%), seizures (0.7%), multiorgan failure (0.7%), Pre syndrome (0.7%), sepsis (0.7%). In the PPI group, we found that during this admission, patients were diagnosed with myocardial infarction (1.26%), pulmonary embolism (3.1%), AKI (2.5%), clostridium difficile (1.26%), Pres syndrome (0.6%), sepsis (0.6%), and cardiac arrest (2.5%).
Discussion
Length of hospital stay in relation to PPI
See Figure 1. Patients who were on PPI before admission were found to have spent fewer days in the hospital, on average, than patients not taking PPI before admission. This is interesting as patients on PPI spending fewer days hospitalised may coincide with current hypotheses that PPI use has potential benefits in COVID-19-infected patients. Lysosomotrophic agents, such as PPIs, can naturally conduct antiviral action by affecting the mechanism of endolysosomal transit by mediating the pH. More studies are required to determine if PPIs may provide positive clinical and therapeutic results in treating patients suffering from COVID-19 infection. As such, PPIs may be helpful not just in the treatment of COVID-19 but in the prophylaxis of it also [21,22]. Although this study did not analyse the types of PPIs taken, it is noted that a German study on omeprazole evidenced its interference with the viral formation of COVID-19 at sub-therapeutic concentrations. It was found that it also elevated the anti-covid-19 properties of aprotinin, as well as Remdesivir, by significant amounts (3-fold and 10-fold, respectively) [23,24]. Most patients in both sub-groups in our study had a hospital stay between 6-15 days. This supports the finding of a Chinese-based retrospective study concluding that they found no overall effect of PPI regimens on adults who were hospitalised with COVID-19 infection, both in evidencing a shorter or more prolonged duration of illness [25]. More patients in the non-PPI group stayed longer in the hospital (>15 days) than patients taking PPIs. It is important to note that a more in-depth review of patient complications and the level of care required (ward-based, HDU, ICU) provides a more accurate representation of disease progression in patients, rather than only considering the length of hospital stay.
Covid-19 outcomes in the PPI group vs non-PPI group
See Figure 3. The mortality rate of PPI group patients was higher: 22.6% in the PPI group vs 19.3% in the non-PPI group. However, this figure was found to be statistically insignificant. A similar but more pronounced finding was discovered in an American retrospective cohort study at a tertiary centre in New York, concluding that there was a more than double effect on mortality when comparing patients taking PPIs vs those not taking PPIs (34.8% vs 16.2%) [8]. There was no association between the mortality rates of both groups of patients. More COVID-19 patients on PPI pre-admission were admitted to HDU (30.8%) compared to a lower 20% in patients not on PPI. Also, more than half of patients taking PPI (56.6%) were admitted to ICU compared to a lower 45.3% of patients not on PPI prior to admission. This is supported by a Korean-based larger retrospective cohort study that showed individuals taking PPIs at the time of the study demonstrated an elevated chance of adverse severe clinical affectation of COVID-19 [12].
ASA scores of patients both on PPI and not on PPI prior to admission
See Figure 5. More than half of the patients taking PPI (57%) had a high ASA score of 3 (indicating severe systematic illness) compared to 46% of patients not taking PPI with an ASA score of 3. This result is in keeping with studies postulating that COVID-19 patients taking PPI have a propensity to more adverse outcomes if infected with COVID-19 [12].
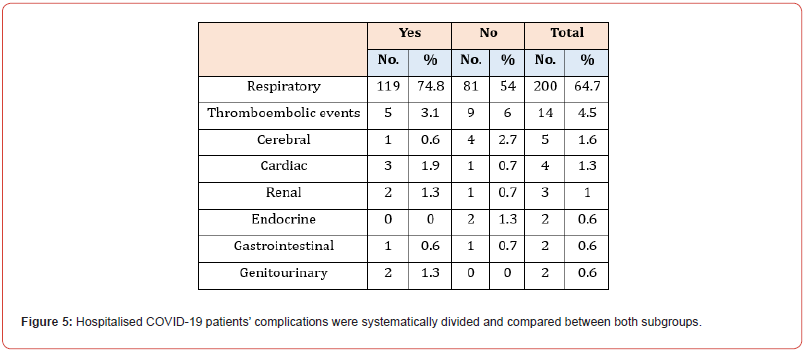
Complications of hospitalised COVID-19 patients were systematically divided and compared between both subgroups
See Figure 5. SARS-COV’s primary consequence was respiratory, which was seen more in patients taking PPI than in non-PPI groups. The most prevalent respiratory consequence developed in both groups was COVID-19 pneumonitis. Regarding a possible cause for the primary respiratory consequence of SARS-Cov, seen more in PPI-taking patients, we can consider an observational cohort study conducted to establish a causal link between PPI usage and resulting secondary infection in patients hospitalised due to COVID-19 infection. PPIs impair the production of gastric acid, and this causes a bacterial build-up in the gastrointestinal tract. A pneumonic process occurs when aspiration occurs and leads to the colonisation of bacteria in the lung. The data gathered showed that just under 50% of individuals on PPI therapy developed a secondary infection compared to a much lower 20% in individuals not on PPI therapy. Following adjustment for external risk factors, the difference in secondary infection development between taking PPI and not was statistically significant [20]. However, regarding ARDS, our study found that more patients who were not taking PPI developed ARDs as a respiratory complication compared to patients who were taking PPI. This finding differs from the retrospective study in that it evidenced a heightened probability of developing ARDS when taking PPI therapy compared with not taking PPI therapy [20].
Conclusion
We found that PPI usage in COVID-19-positive patients at index hospital admission showed worsening outcomes, similar to other published studies. However, it must be reinforced that these studies are observational and thus, causality cannot be established. This proposed causation needs further evaluation via well-conducted prospective studies.
References
- (2022) Who coronavirus (COVID-19) dashboard [Internet]. World Health Organization. World Health Organization.
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (covid-19). JAMA 324(8): 782.
- Shi S, Qin M, Shen B, Cai Y, Liu T, et al. (2020) Association of cardiac injury with mortality in hospitalised patients with COVID-19 in Wuhan, China. JAMA Cardiology 5(7): 802.
- Mao L, Jin H, Wang M, Hu Y, Chen S, et al. (2020) Neurologic manifestations of hospitalised patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology 77(6): 683.
- Mao R, Qiu Y, He JS, Tan JY, Li XH, et al. (2020) Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology 5(7): 667–678.
- Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, et al. (2020) Registry of arterial and venous thromboembolic complications in patients with COVID-19. Journal of the American College of Cardiology 76(18): 2060–2072.
- Vesper B, Jawdi A, Altman K, Haines III G, Tao L, et al. (2009) The effect of proton pump inhibitors on the human microbiota. Current Drug Metabolism 10(1): 84–89.
- Ramachandran P, Perisetti A, Gajendran M (2022) Prehospitalisation proton pump inhibitor (PPI) use and clinical outcomes in COVID-19. Eur J Gastroenterol Hepatol 34(2): 137-141.
- Charpiat B, Bleyzac N, Tod M (2020) Proton pump inhibitors are risk factors for viral infections: even for COVID-19? Clin Drug Investig 40: 897-899.
- Fossmark R, Martinsen TC, Waldum HL (2019) Adverse effects of proton pump inhibitors—evidence and plausibility. International Journal of Molecular Sciences 20(20): 5203.
- Fatima K, Almas T, Lakhani S, Jahangir A, Ahmed A, et al. (2022) The use of proton pump inhibitors and COVID-19: A systematic review and meta-analysis. Tropical Medicine and Infectious Disease 7(3): 37.
- Lee SW, Ha EK, Yeniova AÖ, Moon SY, Kim SY, et al. (2020) Severe clinical outcomes of COVID-19 associated with Proton Pump Inhibitors: A nationwide cohort study with propensity score matching. Gut 70(1): 76–84.
- Darnell ME, Subbarao K, Feinstone SM, Taylor DR (2004) Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods 121(1): 85-91.
- Hayase Y, Tobita K, Sato H (2002) Detection of type B influenza virus genes from biopsied gastric mucosa. J Gastroenterol 37: 101-105.
- Fisher L, Fisher A (2017) Acid-suppressive therapy and risk of infec- tions: pros and cons. Clin Drug Investig 37: 587-624.
- Prag C, Prag M, Fredlund H (2017) Proton pump inhibitors as a risk factor for norovirus infection. Epidemiol Infect 145: 1617-1623.
- Vilcu A, Sabatte L, Blanchon T (2019) Association between acute gastroenteritis and continuous use of proton pump inhibitors during winter periods of highest circulation of enteric viruses. JAMA Netw Open 2(11): e1916205.
- Zhou J, Li C, Zhao G (2017) Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv 3(11): 4966.
- Almario CV, Chey WD, Spiegel BMR (2020) Increased risk of COVID-19 among users of proton pump inhibitors. Am J Gastroenterol 65: 1707-1715.
- Luxenburger H, Sturm L, Biever P (2020) Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor? J Intern Med 289(1): 121-124.
- Homolak J, Kodvanj I (2020) Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int J Antimicrob Agents 56(2): 106044.
- Tastemur S, Ataseven H (2020) Is it possible to use proton pump inhibitors in COVID-19 treatment and prophylaxis? Med Hypotheses 143: 110018.
- Bojkova D, McGreig E, McLaughlin KM (2020) SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles. BioRxiv.
- Aguila EJT, Cua IHY (2020) Repurposed GI drugs in the treatment of COVID-19. Dig Dis Sci 65(8): 2452-2453.
- Xiao Yu Zhang, Hai Bing Wu, Ling Y, et al. (2020) Analysis of the effect of proton pump inhibitors on the course of common COVID-19. J Inflamm Res 14: 287-298.
-
Buse Nur Müştekin, Aleyna Şahin and Ayşe Güneş Bayir*. Prospects for Micronutrient Deficiencies and Nutritional Treatment During Chronic Gastritis. Acad J Gastroenterol & Hepatol. 3(4): 2023. AJGH.MS.ID.000568.
-
Nutrition, Gastritis, Helicobacter pylori, Stomach, Smoking, Abdominal pain, Heartburn, Burning sensation, Nausea, Vomiting, Diarrhea, Anti-inflammatory drugs, H. pylori infection, Gastric adenocarcinoma, Intrinsic factor, Achlorhydria, Hypergastrinemia
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






