 Case Report
Case Report
Arterio-venous Malformation Masquerading as Small Bowel Mass; A Rare Presentation of Overt GI bleeding
Kashif Osmani1*, Sana Hussain2, Snehal Sonawane3, Michael Gianarakis1, Saeed Ali2 and Brian Boulay2
1Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA
2Department of Medicine, Division of Gastroenterology and Hepatology, University of Illinois at Chicago, Chicago, IL, USA
3Department of Pathology, University of Illinois at Chicago, Chicago, IL, USA
Kashif Osmani, Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA
Received Date:February 27, 2024; Published Date:March 08, 2024
Abstract
Arteriovenous malformations (AVMs) in the small bowel can present as obscure gastrointestinal bleeding (OGIB), posing diagnostic challenges. We describe a rare case of GI bleeding from an AVM masquerading as a small bowel mass in a 76-year-old female with comorbidities, who presented with fatigue, black stools, and abdominal pain. Initial diagnostic procedures identified a submucosal and ulcerated jejunal mass, raising suspicion for a cancerous growth. Surgical resection with subsequent pathology revealed an AVM with mucosal ulceration. This case demonstrates the diligence needed for thorough evaluation of atypical AVMs due to the high risk of future complications in OGIB presentations.
Abbreviations: AVM: Arteriovenous malformation; OGIB: Obscure Gastrointestinal Bleeding; ESRD: End Stage Renal Disease; EGD: Esophago- Gastroduodenoscopy; DBE: Double Balloon Enteroscopy; VCE: Video Capsule Endoscopy; BAE: Balloon Assisted Enteroscopy; CTE: CT Enterography
Introduction
Obscure gastrointestinal bleeding (OGIB) is characterized by bleeding of unknown origin, persisting despite a thorough evaluation of the upper and lower gastrointestinal tract [1]. It accounts for ~5% of all gastrointestinal bleeds and can present with overt or occult bleeding [2]. In this case report, we present a rare instance of overt gastrointestinal bleeding from an arteriovenous malformation (AVM) masquerading as a small bowel mass.
Small bowel vascular lesions can be classified using the Yano-Yamamoto classification. Type 1 (angioectasia) appear as punctuate or patchy erythemas, consisting of thin, dilated veins prone to bleeding. Type 2 (Dieulafoy’s lesions) involve large arteries protruding through a mucosal defect, presenting with pulsatile bleeding. Type 3 (AVMs) are characterized by arteries and veins aberrantly connected without a capillary bed. Type 4 (congenital intestinal AVMs) are relatively large and appear as mass or polypoid lesions [3]. Real-time endoscopic observations aid in distinguishing between arterial and venous lesions based on the presence of pulsation, a crucial factor in treatment selection and prognosis [4]. However, it is important to note that the endoscopic classification does not always correlate with diagnostic findings, as we see in the present case, necessitating post-operative histopathological evaluation.
The etiology and pathology of AVMs within small bowel vascular lesions remain incompletely understood. Intestinal AVMs can be further categorized by Moore’s classification. Type 1 AVMs are acquired, primarily affect elderly patients, and commonly found in the right colon. Type 2 are congenital, occur in younger patients, and typically affect the small bowel. Type 3 involve the GI system in individuals with hereditary hemorrhagic telangiectasia [5]. The present case is notable for highlighting a small bowel AVM that does not fit the mold of the typical AVM classifications described above.
Case Presentation
A 76-year-old female with history of ESRD on hemodialysis, diverticulosis on colonoscopy 1 year prior, hypertension, diabetes, and hyperlipidemia presented with a three-week history of fatigue, black stools, and left upper quadrant abdominal pain. She denied nausea, vomiting, or NSAID use. Medications included aspirin 81mg daily and Tylenol as needed for abdominal pain. Upon arrival, vital signs stable, however digital rectal examination revealed melena. Laboratory tests were significant for hemoglobin of 5.7 g/dL (baseline ~10 g/dL), platelet count of 174x10^3/μL, BUN of 27 mg/ dL, creatinine of 3.31 mg/dL, lactate of 1.2 mmol/L, hsTroponin of 14 ng/L, and INR of 0.9. Patient received two units of packed red blood cells and was started on Protonix drip.
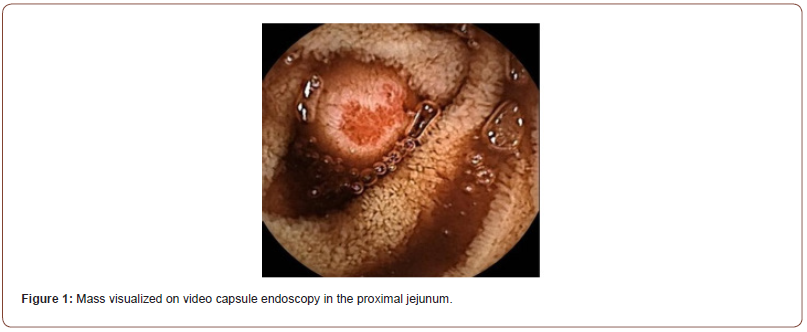
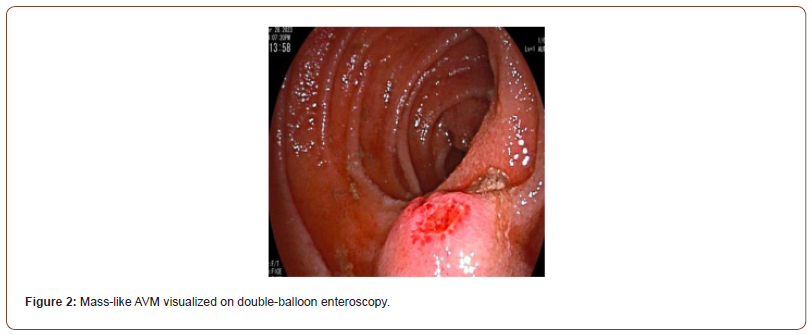
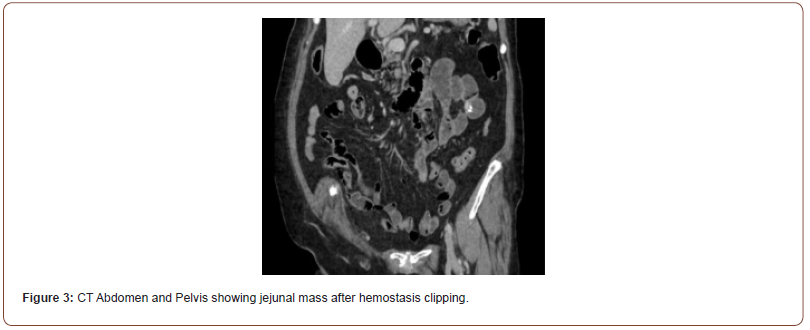
Initial workup consisted of an esophagogastroduodenoscopy (EGD), which revealed mild erythematous mucosa without bleeding at the incisura, gastric antrum, and pylorus. Biopsies with further pathology results were unremarkable. A follow-up video capsule endoscopy demonstrated a ~1cm mass in the jejunum with ulceration at the tip and stigmata of recent bleeding (Figure 1). Subsequently, a double balloon enteroscopy (DBE) confirmed a 13mm submucosal ulcerated mass in the proximal to mid-jejunum (Figure 2). Biopsies of the mass were taken using a cold forceps for histology, which triggered brisk bleeding. Hemostasis was achieved by placing two hemostatic clips. Given the presence of the jejunal mass and the uncertainty surrounding its nature, the surgical oncology team was consulted. A CT abdomen and pelvis was then performed, showing focal enhancement and wall thickening with adjacent clips in a loop of bowel, likely corresponding to the recently biopsied jejunal mass (Figure 3). Based on the presentation and imaging findings suggesting a cancerous mass, surgical resection was performed. The jejunal mass and two associated lymph nodes in the surrounding mesentery were excised without complications.
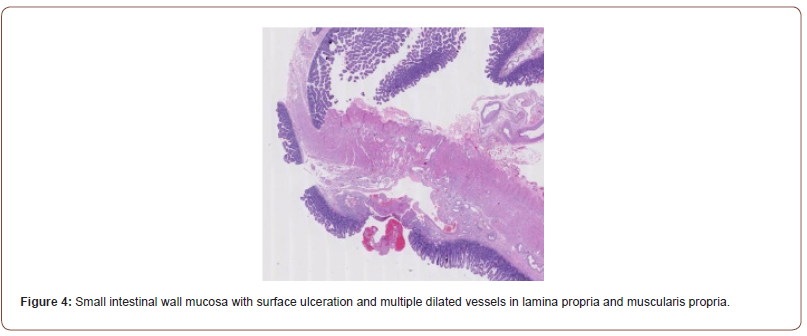
The DBE biopsy showed no evidence of celiac disease, viral cytopathy, metaplasia, parasites, neoplasia, or dysplasia. Gastrointestinal stromal tumor and neuroendocrine tumor were also ruled out. The subsequent bowel resection biopsy revealed dilated blood vessels in the mucosal and submucosal layers, consistent with angiodysplasia/vascular ectasia or AVM. The resection margins were normal, and reactive lymph nodes were observed (Figure 4). Following surgical resection of the AVM, the patient’s melena symptoms completely resolved during the followup period.
Discussion: OGIB presents diagnostic and management challenges as the underlying cause is often not immediately apparent [6]. There are two subtypes of OGIB: obscure- occult bleeding and obscure-overt bleeding. Obscure-occult bleeding is characterized by positive fecal occult blood testing or iron deficiency anemia without visible signs of bleeding. Obscure- overt bleeding involves recurrent or persistent visible bleeding, such as melena or hematochezia, without a definitive identified source using standard diagnostic tests [7]. Although a comprehensive evaluation, including history, physical, endoscopy, and imaging, is crucial for diagnosing OGIB, they are limited in detecting small bowel lesions.
Fortunately, advancements in non-invasive diagnostic modalities, such as video capsule endoscopy (VCE), balloon-assisted enteroscopy (BAE), spiral enteroscopy, and CT enterography (CTE), have revolutionized the approach to diagnosing and managing OGIB (Pasha et al., 2008). VCE offers a noninvasive assessment of the entire small bowel, while BAE enables deeper examination of the small bowel specifically. Radiologic techniques like CTE, tagged red blood cell scintigraphy, and CT angiography assist in localizing and diagnosing bleeding sources. In cases of persistent overt OGIB despite extensive evaluations, intraoperative enteroscopy is considered as a last resort [8].
Several published case studies highlight the diagnostic and management challenges of OGIB. Gralnek et al. (2005) presented a case of recurrent GI bleeding, utilizing multiple diagnostic modalities to diagnose and surgically resect a carcinoid tumor. Ng et al. [9] reported a case of upper GI bleeding caused by gastric AVM, emphasizing the need to consider AVM as a potential cause. Poon and Poon [10] described a challenging case of massive GI bleeding initially misdiagnosed as a duodenal varix but later identified as a duodenal AVM, underscoring the significance of early angiography and surgical excision in unstable patients. Gong et al. [11] reported a case of jejunal AVM initially mistaken for a gastrointestinal stromal tumor, further highlighting the difficulties in diagnosing AVMs.
Conclusion
In conclusion, a systematic diagnostic workup employing multiple modalities is necessary to identify the underlying cause of OGIB and guide appropriate therapies. This case highlights the importance of considering angiodysplasia in the differential diagnosis in patients with obscure GI bleeding across all age groups, even when initial investigations may be suggestive of a tumor. Timely recognition, accurate diagnosis, and appropriate interventions, including surgical resection as needed, are critical for achieving favorable outcomes in patients with obscure GI bleeding associated with vascular malformations. Small intestinal wall mucosa with surface ulceration and multiple dilated vessels in lamina propria and muscularis propria.
References
- Patel A, Vedantam D, Poman DS, Motwani L, Asif, N (2022) Obscure Gastrointestinal Bleeding and Capsule Endoscopy: A Win-Win Situation or Not?. Cureus 14(7): 27137.
- Bhasin DK, Rana SS (2006) Gastrointestinal bleeding: from overt to obscure. Endoscopy 38(2): 116–121.
- Yano T, Yamamoto H, Sunada K, Miyata T, Iwamoto M, et al. (2008) Endoscopic classification of vascular lesions of the small intestine (with videos) Gastrointest Endosc 67: 169–172.
- Sakai E, Ohata K, Nakajima A, Matsuhashi N (2019) Diagnosis and therapeutic strategies for small bowel vascular lesions. World Journal of Gastroenterology 25(22): 2720– 2733.
- Moore JD, Thompson NW, Appelman HD, Foley D (1976) Arteriovenous malformations of the gastrointestinal tract. Arch Surg 111: 381–389.
- Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, et al. (2008) Double- balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: A meta-analysis. Clin Gastroenterol Hepatol 6(9): 671-676.
- Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, et al. (2000) Development of a capsule for direct visualization of the small bowel: The PillCam SB. Gastrointest Endosc 51(3): AB150.
- Awadie H, Khalifeh Saker N, Bahnan G, Azar C (2022) Management of obscure gastrointestinal bleeding: Current status and future directions. World J Gastroenterol 28(11): 1289- 1305.
- Ng SC, Thomas Gibson S, Harbin LJ, Gupta A, Gould SW, et al. (2009) Gastric arteriovenous malformation: a rare cause of upper GI bleed. Gastrointestinal endoscopy 69(1): 155–156.
- Poon RT, Poon A, Chan AC, Fan ST, Lo CM, et al. (2000) Obscure gastrointestinal bleeding caused by a duodenal arteriovenous malformation initially misdiagnosed as a duodenal varix: report of a case. Surg Today 30(3): 271-273.
- Gong H, Yu H, Liu S, Li Z, Wang X (2014) Jejunal arteriovenous malformation: a case report and literature review. BMC Gastroenterol 14: 110.
-
Kashif Osmani*, Sana Hussain, Snehal Sonawane, Michael Gianarakis, Saeed Ali and Brian Boulay. Arterio-venous Malformation Masquerading as Small Bowel Mass; A Rare Presentation of Overt GI bleeding. Acad J Gastroenterol & Hepatol. 3(4): 2024. AJGH.MS.ID.000570.
-
Arterio-venous Malformation; Masquerading; Small Bowel; GI bleeding; Iris Publishers; Iris Publishers Indexing Sites
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






