 Research Article
Research Article
Source Identification of Organic Pollutants in Wastewater Irrigated Areas of Lahore and Mangamandi; Pollutants Source Identification
Abdul Ghaffar, Principal Scientist, Pakistan Institute of Nuclear Science & Technology (PINSTECH), Islamabad-Pakistan.
Received Date: December 06, 2024; Published Date: January 08, 2025
Abstract
Organic pollutants like DDT, DDE, DCP, TCP, Endrin, and Dieldrin were investigated in the shallow groundwater samples for most densely populated “Lahore” city and its surrounding “Mangamandi” areas of Punjab-Pakistan. In these areas, composite wastewater, mainly consisting of industrial and urban wastewater, is being used for cultivation of crops and vegetables. Solid Phase Extraction (SPE) technique was used to extract the organic pollutants and then analyzed by High Pressure Liquid Chromatography (HPLC) and Gass Chromatogram equipped with Mass Spectrometer (GC-MS). Organic pollutants like DDT, DDE, DCP, TCP, Endrin, and Dieldrin were found in certain samples above the permissible limits. Stable isotopes like 13C, 15N and 18O were applied to assess the source of groundwater contamination. Chemical and isotopic data reveal that contamination of groundwater is mainly due to wastewater irrigation and, to some extent, by seepage through unlined wastewater drains in nearby areas. The wastewater containing organic pollutants used for cultivation is filtered through soil to contaminate the shallow groundwater.
Keywords: Groundwater, Organic pollutants, Chemical and isotopic data, Contamination source
Introduction
Groundwater is significant source of water for many municipal water systems [1]. In urban areas, people mainly rely upon municipal water systems [2]. However, withdrawing water from tube wells and hand pumps is also common practice. In these areas, unnecessary pumping by tube wells and recharge by drain water and agricultural activities are causing groundwater contamination [3-8] The industries are releasing untreated effluents in domestic wastewater drains and consequently posing threat to the quality of ground water resources. Owing to poor wastewater drainage systems and wastewater irrigation practices in area has caused contamination of groundwater sources [9,2,10]. In Pakistan, about 80% of the population is dependent on groundwater for household use [11, 12, Afzal et al. 1999, 13]; 1Access to drinking water is reduced either by a shortage in the quantity of water or by the contamination of the groundwater quality of aquifers (WB 2013). Furthermore, porous soil facilitates contamination of shallow aquifers [14; 15], through seepage phenomena [16]. Lah ore and adjacent areas like “Mangamandi” have huge industrial infrastructure, wide variety of industries like pharmaceuticals, herbicide and pesticide manufacturing industries, chemicals etc. These industries are discharging their effluents directly into wastewater drain without any prior treatment. In many areas of this region, like many other developing countries, wastewater irrigation is normal practice where farmers are using wastewater of drains to irrigate fields for different agriculture activities and consequently making areas more prone to contamination by means of seepage of contaminated water through the soil [17]. Furthermore seepage from wastewater drains is also affecting nearby ground water under hydraulic gradient and lead to contamination [18, 19]. The shallow aquifer of the Lahore and Mangamandi area is contaminated in respect of nitrate of high concentrations [20].
Present research work was designed to investigate the impact of wastewater irrigation and wastewater drains on shallow and shallow to deep groundwater of region. The groundwater samples were collected from nearby wastewater drain residential areas and agriculture land being irrigated by wastewater. Groundwater in Lahore and Mangamandi areas. Samples were analyzed for organic pollutants and stable isotopes (δ13C, δ18O and δ15N) to evaluate the impact of wastewater irrigation and wastewater drains possible recharge to groundwater channels. Using characteristic of δ18O and δ15N, the nitrate supplied by precipitation can be distinguished from the nitrate produced by microbial activity in soils or added to soil as fertilizer. Nitrification of ammonium and/or organic-N in fertilizer, precipitation, and organic waste can produce a large range of d values. The data of δ13C was used to evaluate the wastewater mixing.
Materials and Methods
Sampling
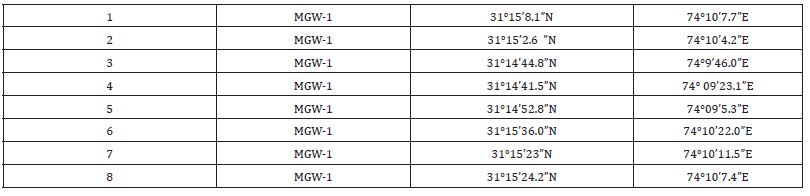
Samples were collected from shallow aquifer with depth less than 100m and greater than 120m referred as shallow to deep groundwater [21]. The sampling points were chosen on the basis of previous studies on organic profile of drains [22]. Sampling points were categorized basis on deep water and shallow water aquifers of agricultural, industrial and residential areas. Groundwater samples were collected from hand pumps, boreholes and tube wells in duplicate in pre-cleaned air tight polypropylene bottles in the month of May-June 2022 (pre-monsoon). Before collecting the water samples, the water was pumped out from bore holes in enough quantity to remove lines stagnant water. Co-ordinates of groundwater samples collected from Lahore (LGW) and Mangamandi (MGW) as given in Table 1 & 2.
Table 1: Coordinates of Saming points of Lahore areal.
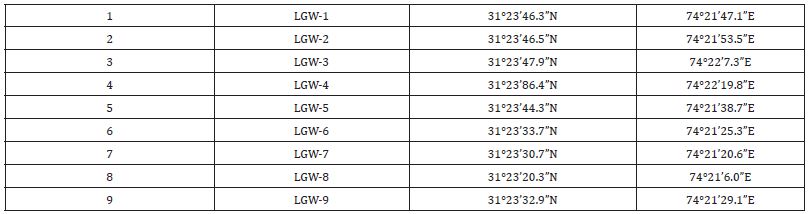
Table 2: Coordinates of Sampling points of Mangamandi area.
Quality parameters like clarity, odor, and color were recorded in the field. All the samples were colorless and odorless as they were being used for drinking purpose. Physicochemical parameters such as the pH, electrical conductivity (EC) and total dissolved solids (TDS) of samples were measured in situ. Dilute HNO3 was added to each sample until the pH was <2 for major cations, then the sample bottles were stored at about 40C. Measurement of pH was done using a digital pH meter (Adwa, Model AD1030). The EC and TDS of collected samples were measured with a portable conductivity meter (WTW-Model LF 95) calibrated with standard solutions from Hanna instruments (Italy). Water samples were filtered through 0.45 μm membrane filter using filtration assembly equipped with vacuum system. The groundwater samples were pre-concentrated prior to analyze on GC-MS and HPLC. Pre-concentration of samples was carried out by using solid phase extraction method using C18 cartridge (Supelco) and elution was carried out using extra pure solvents like aceton, hexane and ethyl acetate (4 ml each) with flow rate of 1mL/Min [23].
Analysis and Measurement
Organic compounds were analyzed using HPLC-UV (Waters 1525) for qualitative analysis and a GC-MS (HP 5890; Hawlett Packard series II) equipped with DB-5m column (30m x 0.25m x 1μm) and a quardrupole mass spectrometer (JEOL). Ionization was performed under 70eV electron impact conditions (300μA, 400V) where the initial temperature of the column was 35oC, raised at 15oC/min to 150oC and then at 3oC/min to 280oC for quantitative analysis.
The stable isotope analyses were performed by using a modified Varian Mat GD-150 Mass Spectrometer. The ammonium distillation method was used to measure the nitrogen isotopes of nitrate in water sample (Sigman et al., 1997). This method involves the reduction of nitrate to ammonium, which is distilled and concentrated as an ammonium sulphate salt and then combusted to produce nitrogen gas for isotope measurement using isotope ratio mass spectrometer (IRMS). For 13C isotope analysis on mass spectrometer, total dissolve inorganic carbon in water samples was converted into gaseous phase. Sample was poured into the pyrex reaction flask and reacted with H3PO4 acid [24]. The reaction flask assembly was connected to the vacuum line. About 5 ml H3PO4 was added to the pre-evacuated reaction flask. The CO2 gas was evolved as a result of reaction between inorganic carbon component/fraction of sample and the phosphoric acid as shown in equation:
H3PO4 + Carbonate mineral→ CO2 + H2O
The moisture produced during the reaction was removed by cryogenic trap of -800C. The CO2 gas was solidify in liquid nitrogen cryogenic trap. Other undesired gases were evacuated to get pure CO2. The pure CO2 was collected in ampoule for 13C analysis on Varian Mat GD-150 Mass Spectrometer. Isotope ratio (δ‰) of 15N, 13C and 18O were calculated by using following relation:

The overall analytical errors ±0.01 ‰ (δ13C & δ18O ) and ±0.1‰ (δ14N) were recorded for measurements. To ensure precision, standard deviation of the mass spectrometer has been computed and the standard deviation of each sample has been ensured to be within permissible limit.
Results and Discussion
Physico-chemical parameters
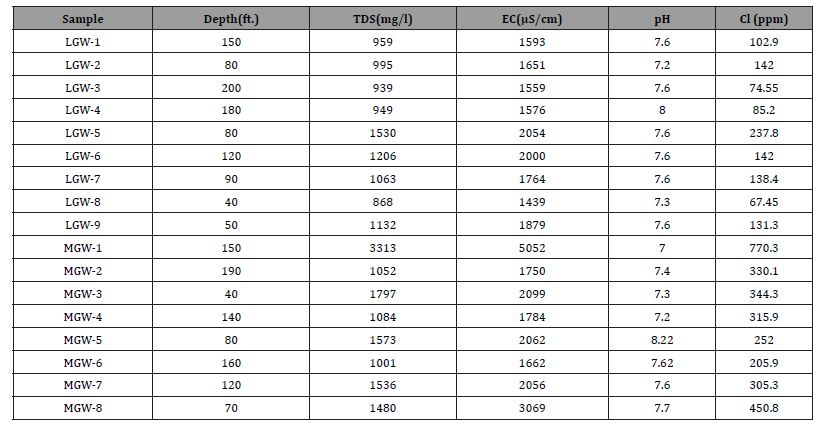
In Lahore samples TDS was found between 939 to 1530mg/L and EC varied between 1439 and 2054 μS/cm. While, in Mangamandi TDS values ranged between 1001 to 3313mg/L whereas, EC values were observed between 1662 to 5052 μS/cm (above the permissible limits) as given in Table 3. Ground water with a TDS above 500 mg/L and EC >2.25 to 4mS/cm is considered not safe for consumption [25].
Table 3: Physiochemical parameters of groundwater samples.
Analysis of Organic Compounds in Lahore Groundwater
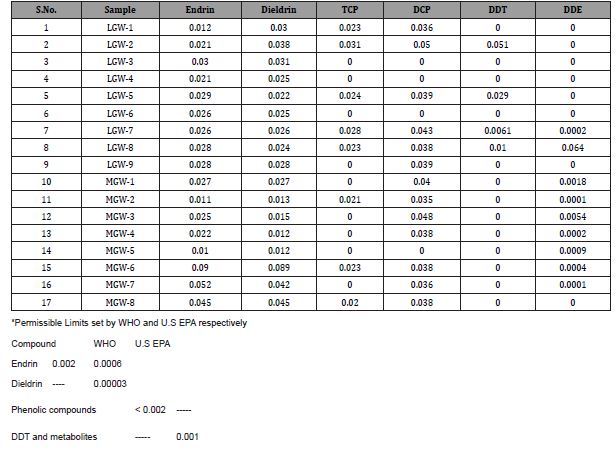
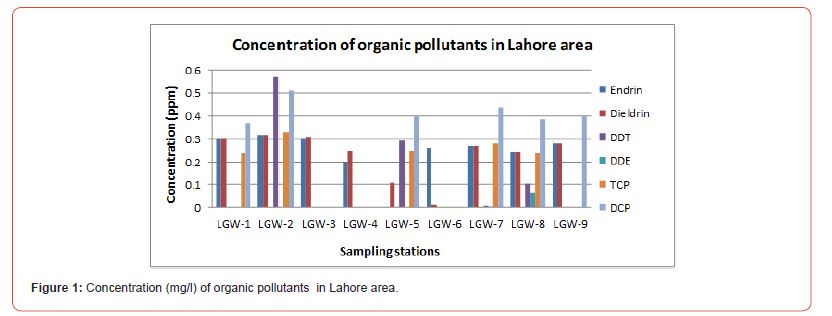
The concentration of organic pollutants in Lahore area is shown in Table 4 and Fig.1. Sample LGW-1 collected from borehole in industrial areas alongside the waste water drain showed high concentrations of Endrin, Dieldrin and DCP. The LGW-2 sample collected from shallow ground water in industrial area near the waste water drain showed concentrations of DCP, TCP, DDT, Endrin and Dieldrin. Both points LGW-1 and LGW-2 were located in the industrial areas along the drain but high concentration of organic pollutants in shallow groundwater sample (LGW-2) as compare to deep groundwater sample (LGW-1) suggested that shallow groundwater channel is more vulnerable to contamination.
Endrin and Dieldrin were found in LGW-3 and LGW-4 samples which were collected from agricultural area near the wastewater drain (upstream). Whereas, LGW-5, sample collected from agricultural area at wastewater drain (downstream) showed e TCP, DCP, Endein, Dieldrin and DDT, Presence of organic contaminants in deep ground water sample (LGW-3) depicts the contamination of soil has reached to its saturation point and contaminants are seeping down in deep water aquifers.
In LGW-6 only Endrin and Dieldrin were recorded, this sample was collected from industrial area whereas more organic pollutants were found in LGW-7, LGW-8 and LGW-9 as these samples were collected from agricultural area along the wastewater drain. The results suggested that shallow groundwater channel in agricultural areas is being more affected by organic contamination, as compare to industrial area, due to wastewater irrigation.
Overall Endrin and Dieldrin were found in nine, DCP in six, TCP in five, DDT in four and DDE in two samples. Contamination of both shallow groundwater (high level) and deep groundwater (low level) is an alarming situation. Shallow groundwater channels are being suffered from wastewater irrigation infiltration and seepage of unlined wastewater drains. Deep Groundwater channels contamination suggested the overloading of organic pollutants on deep soil through wastewater irrigation where the buffering and degradation potentials of soils exhibited low organic carbon retention which might be due to variable and changing nature of organic matter and clay contents in soils [26].
Table 4: Concentrations of organic pollutants in groundwater samples.
Analysis of Organic Compounds in Mangamandi Groundwater
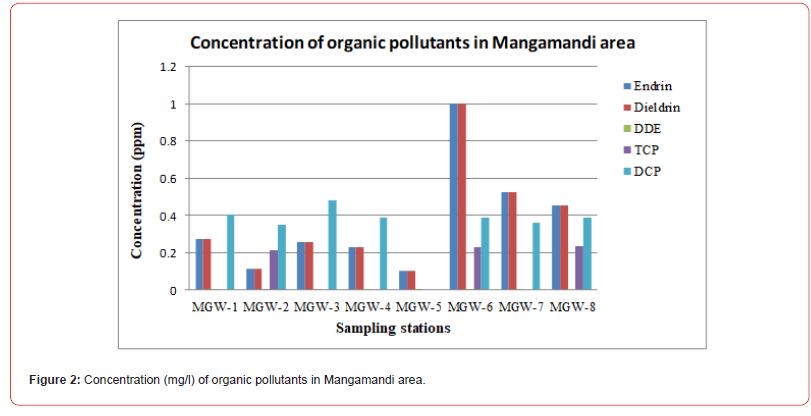
The concentration of organic pollutants in Mangamandi area is shown in Fig. 2. MGW-1 sample was collected from borehole in agriculture area located near the waste water drain whereas MGW-2 sample was collected from borehole located in industrial area on opposite side of drain. It is important to mention that there was agricultural land at one side of the drain and industrial area at the other side. High concentration of organic pollutants in agricultural areas (MGW-1) as compare to non-agricultural area (MGW-1), within same grid, advocate more vulnerability of groundwater channel in agricultural areas towards organic pollutants.
MGW-3 9shallow groundwater sample) was collected from agricultural area near wastewater drain whereas MGW-4 was also collected from same area but it was deep water sample. Comparative high concentration of organic pollutants in MGW-3 as compare to MGW-4 clearly suggest that impact of wastewater irrigation become more worst from shallow to deep groundwater channels.
MGW-5 (shallow groundwater) and MGW-6 (deep groundwater) sample was collected from residential area surrounded by agricultural land. The presence of comparative high concentration of organic pollutants in MGW-5 as compare to MGW-6 indicates shallow groundwater deterioration. MGW-7 and MGW-8 samples were collected from industrial area near agricultural land. Endrin, Dieldrin, DDE and DCP were detected in these samples as well. It might be due to mobility of organic pollutants along groundwater flow patterns.
Overall it was observed that Endrin and Dieldrin were found in eight samples, DCP and DDE in seven, TCP in three samples. It might be due to infiltration of wastewater along with pesticides to contaminate the groundwater [27]. Contamination of even shallow to deep ground water shows that soil has reached to its saturation point and contaminants are seeping down into ground water for its contamination. It is fact that the soil provides a potential pathway of pesticide transport to contaminate water, through runoff and subsurface drainage; interflow and leaching [28-30].
δ13C values in Groundwater Samples
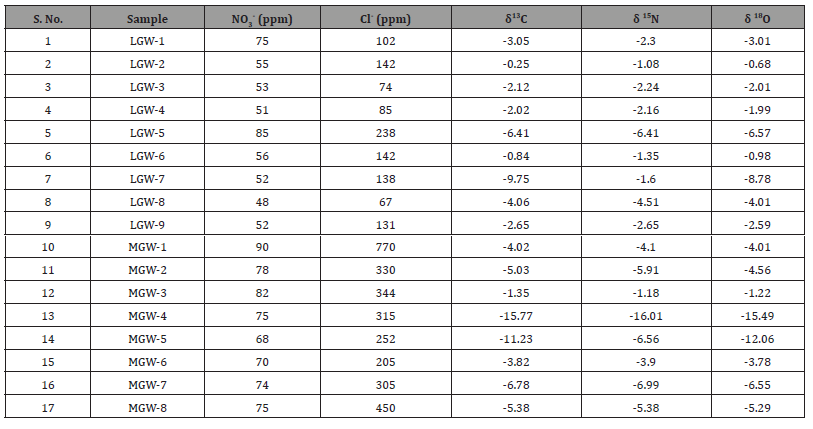
Lahore groundwater samples showed δ13C values ranged from -0.25 to -6.41 ‰ PDB. In Lahore samples, LGW-1 was collected from deep borehole and its value -6.35‰. The δ13C depleted values shows that deep water has wastewater mixing. LGW-2 was collected from shallow water and it showed less depleted δ13C values, it indicates that this point has almost no waste water mixing. LGW-3 was collected from borehole and it showed slightly depleted values which suggests that this water is slightly affected by wastewater. LGW-4 (deep channel) have minor depleted δ13C‰ values, however LGW-5 (deep channel) showed depleted δ13C ‰ values. It suggested that shallow groundwater channels has more wastewater mixing. LGW-6 (shallow channel) has ignorable depleted values which might be due to sufficient aeration by plant roots respiration or oxidation conditions. LGW-7 and LGW- 8 were collected from shallow water and depleted δ13C ‰ values suggest wastewater mixing. LGW-9 was collected from hand pump (shallow water) its more depleted δ13C ‰ value proves more wastewater mixing.
In Mangamandi samples, MGW-1 and MGW-2 were collected from deep water (borehole) it showed depleted δ13C ‰ values which suggest that these groundwater samples have wastewater mixing. MGW-3 was collected from shallow wastewater channel and has negligible depleted δ13C‰ values due to plant roots respiration or prevailing oxidation conditions. MGW-4 and MGW-5 have higher depleted δ13C‰ which suggest higher wastewater mixing. MGW-6 (deep channel) showed less depleted δ13C‰ values due to less wastewater mixing. MGW-7 and MGW-8 were collected from shallow water and they have depleted δ13C‰ values, which points towards wastewater mixing.
Table 5: Isotopic values (δ ‰) of groundwater samples.
Source of contamination in Groundwater
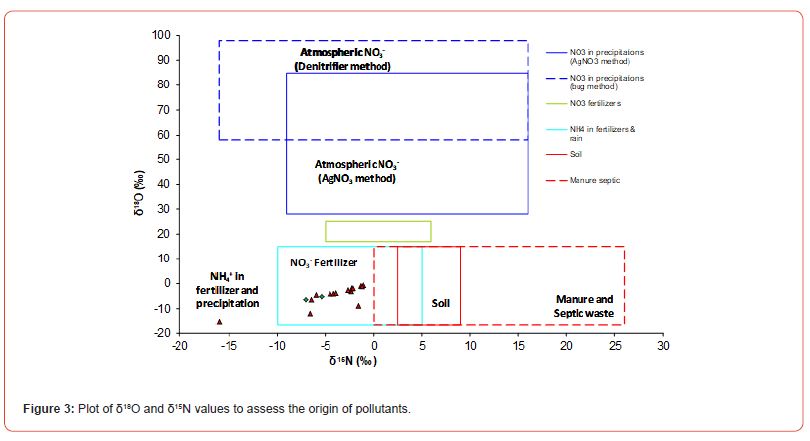
The source of pollution in the groundwater was assessed by using the stable isotope tools. Ratio of δ18O and δ15N were applied to determine the source of pollutants as shown in Fig. 3.
The ratios of δ18O and δ15N of nitrates in water samples were placed in the assigned plot values of Kendall and source of organic pollutants induced in groundwater of Lahore and Mangamandi were assessed. The sources of pollutants assessed at sample points based on isotopic data are anthropogenic source and that nitrates in groundwater is coming mainly from fertilizers that were being used in fields. Nitrates of these fertilizers seeped through the soil and become part of groundwater along with wastewater that is used for cultivation.
Conclusion
a. Significant concentration of organic compounds including Endrin, Dieldrin, DCP, TCP, DDE, DDT was found in groundwater sample of Lahore and Mangamandi areas.
b. Shallow groundwater channels of Lahore and Mangamndi areas are being contaminated mainly due to wastewater irrigation and, to some extent, through seepage from wastewater drains passing through the area.
c. The ratios of δ18O and δ15N of nitrates in water samples suggested that nitrates in groundwater is mainly coming from fertilizers being used in fields.
d. The depleted δ13C‰ value in some areas in vicinity of wastewater drains suggested that wastewater drains, at some extend, contributing to contaminate the shallow groundwater channels.
e. The shallow groundwater channels in the areas of Lahore and Mangamandi, are under high risk due to filtration caused by wastewater irrigation and seepage of unlined wastewater drains flowing through the areas.
f. Wastewater irrigation in Lahore and Mangamandi areas should be banned and wastewater drains should be lined to conserve the quality of groundwater.
Conflicts of Interest
None.
Acknowledgement
None.
References
- Bralower T, Bice D (2014) Distribution of Water on the Earth’s Surface, College of Earth and Mineral Science, The Pennsylvania State University.
- Rail, Chester D (2000) Groundwater contamination, volume 1: Contamination, sources, & hydrology. Lancaster, Pennsylvania: Technomic Publishing Company, Inc.
- NESPAK (1991) Contribution of private tube wells in development of water potential. Final Report, Ministry of Planning and Development, Islamabad.
- Zuberi FA, Sufi AB (1992) State of art of groundwater exploration, exploitation, management and legislation. IWASRI, WAPDA, Lahore, 26 -45.
- World Health Organization, Guidelines for drinking-water quality: incorporating first addendum. Vol. 1, Recommendations. 3rd ed., ISBN 92 4 154696 4.
- Ahmad S (1993) Viability of agriculture resource base: A critical appraisal. In agricultural Strategies in the 1990s: Issues and options. Pakistan Association of Agricultural Social Scientists, Islamabad p. 449-466
- Ahmad S, Mulk S, Amir M (2002) Groundwater Management in Pakistan’. In First South Asia Water Forum Kathmandu Nepal. Printed by Pakistan Water Partnership.
- Yeping M, Miao L, Miaomiao W, Zhen L, Xiang L (2015) Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Science of the Total Environment 51 (8): 498-506.
- Oren O, Yechieli Y, Böhlke JK, Dody A (2004) Contamination of groundwater under cultivated fields in an arid environment, central Arava Valley, Israel. Journal of Hydrology 290(3): 312-328.
- Chilton J, Chapman D (1996) Water Quality Assessments - A Guide to Use of Biota, Sediments and Water in Environmental Monitoring - Second Edition, UNESCO/WHO/UNEP,ISBN 0 419 21590 5 (HB) 0 419 21600 6 (PB).
- WWF (2007) Pakistan’s Waters at risk: Freshwater & Toxics Programme,” WWF-Pakistan.
- PCRWR (2002) Water quality status in Pakistan, 1st report 2001-2002, Pakistan Council of Research in Water Resources.
- Bhutta MN (1999) Vision on water for food and agriculture: Pakistan’s perspective. Regional South Asia Meeting on Water for Food and Agriculture Development. New Delhi.
- Tariq MI, Afzal S, Hussain I (2004) Pesticides in shallow groundwater of Bahawalnagar, Muzaffargarh, DG Khan and RajanPur districts of Punjab, Pakistan. Environment international 30(4): 471-479.
- Chester D, Rail (2000) Groundwater contamination, volume 1: Contamination, sources, & hydrology. Lancaster, Pennsylvania: Technomic Publishing Company, Inc.
- Kanwal S, Gabriel HF, Mahmood K, Ali R, Haidar A, et al. (2015) Lahore's Groundwater Depletion-A Review of the Aquifer Susceptibility to Degradation and its Consequences, Technical Journal, University of Engineering and Technology (UET) Taxila, Pakistan, 20 (I): 2015.
- Ahmad M, Rafiq M, Akram W, Tasneem MA, Ahmad N, et al. (2002) Assessment of aquifer system in the city of Lahore, Pakistan using isotopic techniques , IAEA Report, XA0202352.
- Malarkodi M, Krishnasamy R, Kumaraperumal R, Chitdeshwari T (2007) Characterization of heavy metal contaminated soils of Coimbatore district in Tamil Nadu. Journal of Agronomy 6(1): 147-151.
- Deepali GK, Gangwar K (2010) Metals concentration in textile and tannery effluents, associated soils and ground water. New York Science Journal 3(4): 82-89.
- Khan MS, MH Malik (2000) Effects of biological contamination on the water quality of Wah Cantt. Area Pakistan. GEOSAS-111, Lahore.
- Wang S, Tang C, Song X, Yuan R, Wang Q, et al. (2013) Using major ions and δ15N–NO3− to identify nitrate sources and fate in an alluvial aquifer. Environ Science Process Impacts 15: 1430-43.
- Sumera N (2013) Qualitative and quantitative analysis of organic pollutants in Industrial Wastewater used for agriculture purposes in Pakistan. International Islamic University, Islamabad, MS thesis, Reg. 117-FBAS/MSES/F-11.
- Tanabe A, Mitobe H, Kawata K, Sakai M, Yasuhara A (2000) New monitoring system for ninety pesticides and related compounds in river water by solid-phase extraction with determination by gas chromatography/mass spectrometry. J AOAC Int . 83(1): 61-77.
- McCrea JM (1950) On the isotopic chemistry of carbonate and paleotemperature scale. Journal of Chemical Physics 18: 849-859.
- Thomas H (2003) Groundwater Quality and Groundwater Pollution. University of California, Division of Agriculture and Natural Resources: FWQP Reference Sheet 11(2).
- Tariq MI, Afzal S, Hussain I, Sultana N (2007) Pesticides exposure in Pakistan: A review. Environment International 33(8): 1107-1122.
- Turberg P, Müller I, Flury F (1994) Hydrogeological investigation of porous environments by radio magnetotelluric-resistivity (RMT-R 12–240 kHz). Journal of Applied Geophysics 31(1): 133-143.
- Abrahams PW (2002) Soils: their implications to human health. Science of the Total Environment 291(1): 1-32.
- US EPA (2015) Secondary Drinking Water Standards.
- Wauchope R (1992) Environmental risk assessment of pesticides: improving simulation model credibility. Weed Technology 5(1): 753-759.
-
Abdul Ghaffar*. Source Identification of Organic Pollutants in Wastewater Irrigated Areas of Lahore and Mangamandi; Pollutants Source Identification. Adv in Hydro & Meteorol. 2(3): 2025. AHM.MS.ID.000540.
-
Wastewater; Organic pollutants; Irrigated areas; Crops; Vegetables; Groundwater; Tube wells; Hand pumps; Isotopic data; Organic compounds; Water resources; Soil; Farmers
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






