 Case Report
Case Report
Thoracic Intramedullary Mass Causing Neurologic Weakness
Jonathan B Wallach1*, David L Schwartz1, Sarah P Mattessich1 and Parinda N Shah2
1Department of Radiation Oncology, VA New York Harbor Medical Center, SUNY Downstate Medical Center, USA
2Department of Radiology, VA New York Harbor Medical Center, New York University School of Medicine, USA
Jonathan B Wallach, Department of Radiation Oncology, VA New York Harbor Medical Center, SUNY Downstate Medical Center, USA
Received Date: June 12, 2024; Published Date: July 05, 2024
Case Description
An 87-year-old man with a past medical history of hyperlipidemia, hypertension, benign prostatic hyperplasia, chronic obstructive pulmonary disease, low-grade right urothelial carcinoma status post biopsy two years prior, and atrial fibrillation status post prior cardioversion six years earlier not on anticoagulation presented to the emergency room reporting one month of right lower extremity weakness, progressing to an inability to ambulate. He also reported severe right groin pain and increasing urinary obstruction.
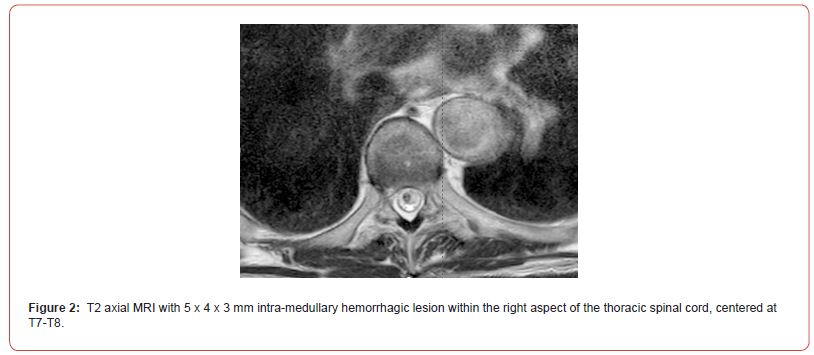
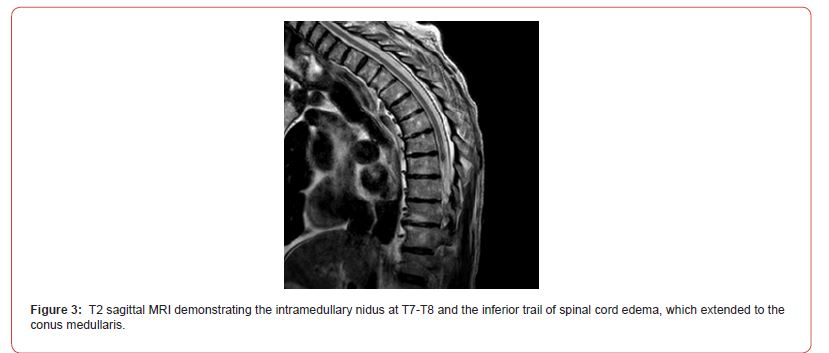
On admission, Neurology evaluated his lower extremity strength as 5/5 on the left, but on the right, was 1/5 at the hip and 2/5 at the knee, with hypoesthesia of the right lower extremity. A Computed Tomography (CT) of the chest/abdomen/pelvis with contrast demonstrated moderate to severe right-sided hydronephrosis, and a proximal right ureteric mass; there was no evidence of systemic metastases. He underwent a Magnetic Resonance Imaging (MRI) of the cervical/thoracic/lumbar spine +/- gadolinium, which showed a mass at T7-T8, mass effect in the central cord, and abnormal spinal cord enhancement from T7 through the conus medullaris (Figures 1-3). An MRI-brain +/- gadolinium did not demonstrate any intracranial metastatic disease, acute infarct, hemorrhage, mass effect, or extra-axial fluid collections.
What is the etiology of this intramedullary abnormality?
A) Intramedullary metastasis
B) Vascular abnormality
C) Intramedullary primary tumor
D) Traumatic spinal injury
Discussion And Conclusions
Lesions involving the spinal cord are traditionally classified by location as extradural, intradural/extramedullary, or intramedullary. Intramedullary spinal cord abnormalities pose considerable diagnostic and management challenges, due to the risks of biopsy in this location, and the added potential for morbidity and mortality for improperly treated lesions. While MRI is the imaging modality of choice, PET/CT and MRA may also serve vital roles in narrowing the differential diagnosis, and potentially avoiding complications from an invasive biopsy [1]. Intradural tumors account for 2-4% of all primary Central Nervous System (CNS) tumors [2]. Ependymomas account for about 50-60% of intramedullary tumors in adults, while astrocytomas account for about 60% of all lesions in children and adolescents [3-4]. The differential for intramedullary tumors also includes hemangioblastoma, metastases, primary CNS lymphoma, germ cell tumors, and gangliogliomas [5,6].
Intramedullary metastases remain rare, although the incidence is thought to be rising. Autopsy studies have demonstrated approximately 2% of patients with systemic cancer have intramedullary metastases. In patients with an established history of malignancy, a metastatic intramedullary tumor should be placed higher on the differential diagnosis. Intramedullary metastases most often occur in the setting of widespread metastatic disease. The vast majority of patients have co-occurring brain metastases and up to 25% have concurrent leptomeningeal metastases. While almost half of all intramedullary metastases are associated with lung cancer, colorectal, breast, renal cell carcinoma, lymphoma, and melanoma can also metastasize to this space.
On imaging, intramedullary metastases often appear as several short, studded segments, with surrounding edema, typically out of proportion to the size of the lesion [1]. By contrast, astrocytoma and ependymomas often span multiple segments and enhancement patterns can vary depending on the subtype and grade. Glioblastoma multiforme (GBM), or grade 4 astrocytoma’s, demonstrate an irregular heterogenous pattern of enhancement. Hemangioblastomas vary in size and are classically hypointense to isointense on T1-weighted sequences, isointense to hyperintense on T2-weighted sequences, and demonstrate avid enhancement on T1-post contrast images. In large hemangioblastomas, flow voids due to prominent vasculature may be visualized.
Numerous non-neoplastic tumor mimics can obscure the differential diagnosis. Vascular abnormalities, including cavernomas and dAVFs, can also present with enhancement and edema. Amongst vascular malformations, dAVFs are the most common type of spinal vascular malformation, accounting for about 70% of cases [7]. They are supplied by the radiculomeningeal arteries, whereas pial arteriovenous malformations (AVMs) are supplied by the radiculomedullary and radiculopial arteries. On MRI imaging, dAVFs usually have venous congestion with intramedullary edema, which is seen as an ill-defined Centro medullary hyperintensity on T2-weighted imaging over multiple segments. As well, the spinal cord may appear swollen with atrophic changes in chronic cases. Spinal cord AVMs are rarer with intramedullary nidus. They usually demonstrate mixed heterogenous signal on T1- and T2-weighted imaging due to blood products, while the nidus demonstrates a variable degree of enhancement. There are serpentine flow voids both within the nidus and at the cord surface.
Demyelinating lesions of the spine may be seen in neuroinflammatory conditions such as multiple sclerosis, neuromyelitis optica spectrum disorders, acute transverse myelitis, and acute disseminated encephalomyelitis. In multiple sclerosis, lesions will typically extend no more than two vertebral segments in length, cover less than half of the vertebral cross-sectional area, and have a dorsolateral predilection [8]. Active lesions may demonstrate enhancement along the rim, or in a patchy pattern. In the presence of demyelinating lesions, there may sometimes appear to be an expansile mass with a syrinx [9].
Infections such as tuberculosis and neurosarcoidosis should also remain on the differential diagnosis. On MRI, tuberculosis would usually involve the thoracic cord and is typically rim enhancing [10]. If there are caseating granulomas, then T2-weighted imaging may demonstrate rim enhancement [11]. Spinal sarcoidosis is unusual without intra-cranial involvement, and its appearance may include leptomeningeal enhancement, expansion of the cord, and a hyperintense signal on T2-weighted imaging [12]. Finally, iatrogenic causes are also possible, including radiation myelopathy and mechanical spinal cord injury. For radiation myelopathy, it is important to ascertain whether a patient has undergone prior radiotherapy in the region, and to obtain the pertinent dosimetry. Spinal cord injury may cause a focal signal abnormality at the cord, with T2 hyperintensity; these foci may or may not present with enhancement, edema, and hematoma, and therefore may resemble tumors [8].
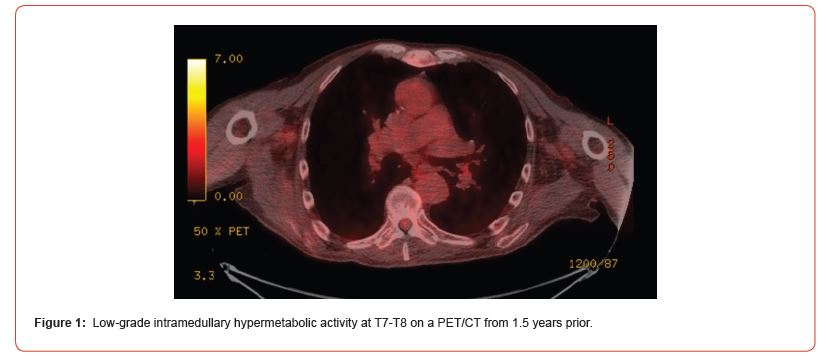
For this man’s clinical presentation with progressive rightsided lower extremity weakness and hypoesthesia, he had a history of a low-grade right renal/pelvic ureteral tumor, so the immediate thought was that the thoracic intramedullary lesion represented a metastatic lesion. However, in the absence of any systemic or intracranial metastases this was much less likely. An extensive work-up was done, through the interdisciplinary work of Medical Oncology, Neurology, Neuro-Oncology, Neuroradiology, Neurosurgery, Nuclear Medicine, and Radiation Oncology. Neuroradiology and Nuclear Medicine discovered the presence of a slightly hypermetabolic focus on a PET/CT from 1.5 years prior that exactly correlated with the same location as the lesion on the recent MRIspine. This finding, as well as his MRA, confirmed the diagnosis of a dAVF, which was successfully managed by conservative measures through dexamethasone and physical therapy, and not through oncologic treatments such as radiotherapy.
There remains a debate as to the utility of steroids in treating patients with dAVF. While there are some case reports documenting edema associated with the dAVF responds to steroids, other case series have found that steroids may worsen outcomes in patients with dAVF, possibly due to increased venous hydrostatic pressure. This case demonstrates the importance of an interdisciplinary work-up when confronted with an intramedullary lesion, as well as maintaining a wide differential diagnosis. As well, all the clues (such as the slightly hypermetabolic focus on a PET/CT from 1.5 years prior) need to be obtained to comfortably reach a diagnosis in the absence of pathologic confirmation. These cases can prove especially challenging due to the unavailability of pathologic confirmation, but in understanding the main differentiating features amongst the various etiologies and obtaining all available information, a correct diagnosis can be made without unnecessary interventions.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
References
- Moghaddam SM, Bhatt AA (2018) Location, length, and enhancement: systematic approach to differentiating intramedullary spinal cord lesions. Insights into Imaging 9(511-526): 12.
- Grimm S, Chamberlain MC (2009) Adult primary spinal cord tumors. Expert Review of Neurotherapeutics 9(10): 1487-1495.
- Miller DJ, McCutcheon IE (2000) Hemangioblastomas and other uncommon intramedullary tumors. J Neurooncol 47: 253-270.
- Mottl H, Koutecky J (1997) Treatment of spinal cord tumors in children. Med Pediatr Oncol 29: 293-295.
- Kandemirli SG, Reddy A, Hitchon P, Saini J, Bathla G (2020) Intramedullary tumours and tumour mimics. Clinical Radiology 75(11): 876 e17-876.e32.
- Tobin MK, Geraghty JR, Engelhard HH, Linninger AA, Mehta AI (2015) Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus 39(2): E14.
- Krings T (2010) Vascular malformations of the spine and spinal cord: anatomy, classification, treatment. Clin Neuroradiol 20: 5-24.
- Maj E, Wojtowicz K, Aleksandra PP, et al. (2019) Intramedullary spinal tumor-like lesions. Acta Radiol 60(8): 994-1010.
- Waziri A, Vonsattel JP, Kaiser MG, et al. (2007) Expansile, enhancing cervical cord lesion with an associated syrinx secondary to demyelination. Case report and review of the literature. J Neurosurg Spine 6: 52-56.
- Nussbaum ES, Rockswold GL, Bergman TA, et al. (1995) Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg 83: 243-247.
- Lu M (2010) Imaging diagnosis of spinal intramedullary tuberculoma: case reports and literature review. J Spinal Cord Med 33:159-62.
- Do-Dai DD, Brooks MK, Goldkamp A, et al. (2010) Magnetic resonance imaging of intramedullary spinal cord lesions: a pictorial review. Curr Probl Diagn Radiol 39: 160-85.
-
Jonathan B Wallach*, David L Schwartz, Sarah P Mattessich and Parinda N Shah. Thoracic Intramedullary Mass Causing Neurologic Weakness. Adv Can Res & Clinical Imag. 4(3): 2024. ACRCI.MS.ID.000589.
-
Computed Tomography; Magnetic Resonance Imaging; Central Nervous System; Glioblastoma multiforme
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






