 Editorial
Editorial
Hypothyroidism, Hypertension and Breast Cancer: Scientific Insights
Amani E Khalifa*
Faculty of Pharmacy, Ain Shams University and Scientific Consultant for Children Cancer Hospital (CCHE 57357), Cairo, Egypt
Amani E Khalifa, Professor of Pharmacology & Toxicology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt.
Received Date: August 05, 2018; Published Date: August 20, 2018
Editorial
Subclinical hypothyroidism is an early stage of hypothyroidism and is usually manifested as elevated thyrotropin-stimulating hormone (TSH) and normal free thyroxine (T4) levels. In such cases, clinical symptoms are usually not manifested although some cases may have minor/subtle symptoms of hypothyroidism and may have mild abnormalities of serum lipoproteins and cardiac function. This condition may resolve, remain unchanged, or develop into overt hypothyroidism within few years, with low free T4 levels as well as a raised TSH level. The likelihood that this will happen increases with greater TSH elevations and detectable antithyroid antibodies [1-5].
Recommendations about thyroid screening, however, have been inconsistent and there is no routine universal screening. The U.S. Preventive Services Task Force for example has recommended that asymptomatic adults not be screened because evidence of clinical benefit is insufficient. Some researchers, on the other hand, have recommended testing in women more than 40 years of age and in patients in geriatric facilities. Yet, other investigators demonstrated that TSH screening every five years, starting at age 35, was cost-effective because progression to overt hypothyroidism was prevented, serum cholesterol levels were reduced and symptoms were relieved with early treatment of hypothyroidism. The relationship among subclinical hypothyroidism, hypertension and breast cancer is of significant importance to many researchers, practicing physicians and their patients. Although hypertension is not a typical sign of hypothyroidism, many studies implicate hypothyroidism as a cause of hypertension. Epidemiological evidence concerning thyroid disorders and breast cancer risk remains unclear. Some epidemiological studies have reported increased risks associated with hypothyroidism, hyperthyroidism, goiter and thyroid autoimmune diseases, while others have found no association. Nevertheless, hypothyroidism was reported to induce the breast epithelial cells’ sensitivity to prolactin and estrogen.
Norepinephrine and the thyroid axis are believed to share important roles in metabolic function, reactions to stress, and affective disorders. More specifically, thyroid hormone produces mental, physical and metabolic effects similar to those of stimulation of noradrenergic receptors but through different mechanisms. Investigators reported reduced noradrenergic activity during hyperthyroidism, possibly mediated by an increase in autoreceptor function. In addition, the sympathetic nervous system, being under the control of the CNS, shows an elevated noradrenaline secretion in organisms with a deficit of thyroid hormones. Therefore, it is hypothesized that prolonged compromised thyroid function may not only result in above normal TSH levels in an attempt to restore normal thyroxine level but also could result in feedback stimulation of noradrenergic transmission through homeostatic mechanisms to reach optimum body functions. Research studies on estrogen-dependent breast cancer revealed that endogenous estrogens can cause cancer, since the estrogens contain a benzene ring in their structure, and benzene and polycyclic aromatic hydrocarbons (PAH) are carcinogenic. More specifically, testing of the endogenous estrogens, estrone (E1) and estradiol (E2), and their catechol demonstrated that they induce tumors in hormonedependent and -independent organs and hence E1 and E2 were classified as epigenetic carcinogens. Moreover, reaction of specific estrogen metabolites, with DNA can generate the critical mutations to initiate breast and other human cancers.
Aromatization of androstenedione and testosterone catalyzed by aromatase enzyme yields E1 and E2, respectively. E1 and E2 are interconverted by the enzyme 17b-estradiol dehydrogenase and are metabolized by two major pathways. The first is the formation of catechol estrogens by hydroxylation at the 2- or 4-position, and the second is the 16a-hydroxylation. CYP1A1 preferentially hydroxylates E1 and E2 at the 2-position, whereas CYP1B1 almost exclusively catalyzes the formation of 4-OHE1 and 4-OHE2. The two catechol estrogens are inactivated by conjugating reactions such as glucuronidation and sulfation, especially in the liver. However, the most common pathway of conjugation in extra-hepatic tissues is O-methylation of the hydroxyl group, catalyzed by the ubiquitous Catechol-O-methyltransferase (COMT). So, COMT is involved in phase II metabolism that leads to protective conjugation of estrogen metabolites or detoxify reactive oxygen species (ROS) formed in these reactions. This conjugation pathway can become insufficient if the activity of COMT is low. In that case, the competitive oxidation of 2-OHE1(E2) and 4-OHE1(E2) to their respective semiquinones and quinones by CYP or peroxidases can increase contributing to the formation of superoxide radicals and depurinating DNA adducts (Figure 1). Genetic variants of genes encoding key proteins of the estrogen metabolic pathway are prime candidates for a possible association with breast cancer risk. In particular, polymorphisms in COMP gene encoding key proteins of estrogen metabolic pathway potentially contribute to breast cancer risk since polymorphic variation in COMT reduces COMT enzyme activity, decreasing the conversion of potentially cancerous estrogens and depurinating DNA adducts into compounds that can then be excreted [6-10].
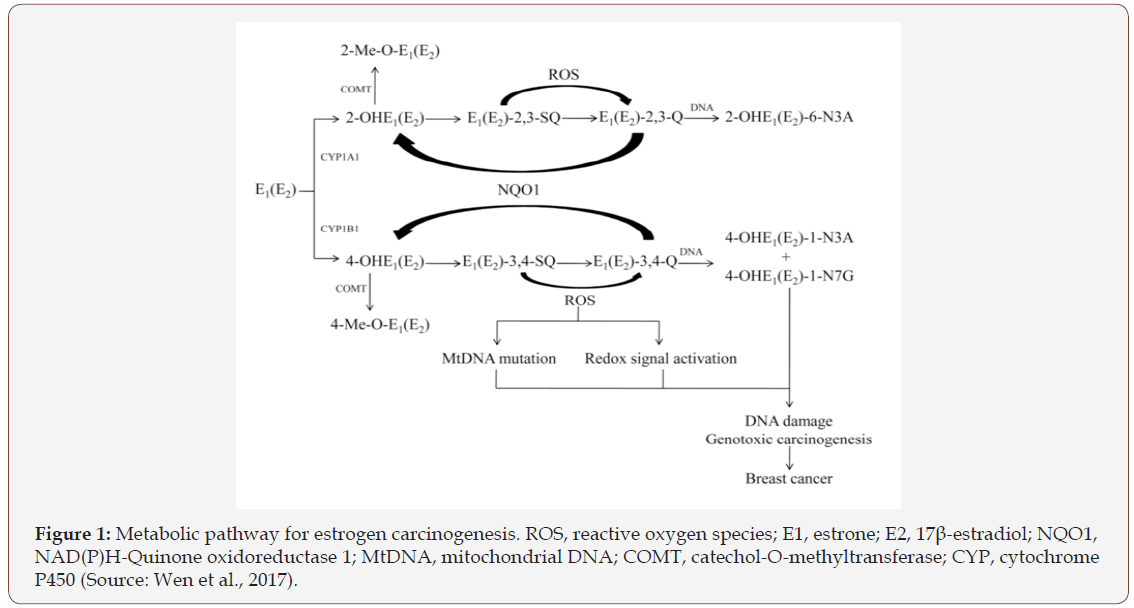
Interestingly, COMT is not only an important enzyme in the metabolism of catechol estrogens but also catecholamines as another catechol structured compounds. Therefore, polymorphic variation in COMT is also expected to raise norepinephrine and epinephrine levels leading to hypertension.
It is therefore believed as an opinion that chronic subclinical hypothyroidism that is left untreated, even with above normal TSH levels lower than 10μU per mL (10mU per L), could potentially predispose to breast cancer and/or severe hypertension as a result of a homeostatic bodily mechanism to restore balance/function by polymorphic variation in COMT that results in producing high levels of noradrenaline/adrenaline, cancerous estrogens and depurinating DNA adducts. Person to person genetic differences and environmental factors may define subpopulations of patients having chronic untreated subclinical hypothyroidism who are at higher risk of developing hypertension and/or breast cancer. Their exposure to other risk factors relevant to the development of hypertension and/or breast cancer should be taken into consideration.
Therefore, in this article, TSH screening of individuals with a family history of hypertension and/or breast cancer is suggested to be performed every five years, starting at age 35, and early treatment before reaching TSH levels of 10μU per mL (10mU per L) may be valuable not only to prevent progression to overt hypothyroidism, reduce serum cholesterol levels and relieve symptoms but also to protect form formation of a COMT variant (as a counter mechanism to enhance noradrenergic function that mimic thyroid hormone function) leading to hypertension and/or breast cancer. Future epidemiologic studies should investigate the percentage incidence of hypertension and/or breast cancer in patients who experienced prolonged untreated subclinical hypothyroidism with or without a family history of hypertension and/or breast cancer.
References
- Chaker L, Bianco AC, Jonklaas J, Peeters RP (2017) Hypothyroidism. Lancet 390 (10101): 1550-1562.
- Hofstetter L, Messerli F H (2018) Hypothyroidism and hypertension: fact or myth?. Lancet 391(10115): 29-30.
- Søgaard M, Farkas DK, Ehrenstein V, Lunde Jørgensen JO, Dekkers OM, Sørensen HT (2016) Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. Eur J Endocrinol 174(4): 410- 414.
- Weng C-H, Chen Y-H, Lin C-H, Luo X, Lin T-H (2018) Thyroid disorders and breast cancer risk in Asian population: a nationwide populationbased case-control study in Taiwan. BMJ Open 8(3): e020194.
- Swann AC (1988) Thyroid hormone and norepinephrine: effects on alpha-2, beta, and reuptake sites in cerebral cortex and heart. J Neural Transm 71(3): 195-205.
- Polikar R, Kennedy B, Maise A, Ziegler M, Smith J, Dittric H, Nicod P (1990) Decreased adrenergic sensitivity in patients with hypothyroidism. J Am Coll Cardiol 15(1): 94-98.
- Nagel-Hiemke M, GroB G, Lues I, Schiimann H-J (1981) Influence of Hypo- and Hyperthyroidism on Plasma Catecholamines in Pithed Rats. Naunyn Schmiedebergs Arch Pharmacol 317(2): 159-164.
- Cavalieri EL, Rogan EG (2010) Depurinating estrogen–DNA adducts in the etiology and prevention of breast and other human cancer. Future Oncol 6(1): 75-91.
- Cerne1 J-Z, Pohar-Perme M, Novakovic S, Frkovic-Grazio S, Stege V, Gersak K (2011) Combined effect of CYP1B1 , COMT , GSTP1 , and MnSOD genotypes and risk of postmenopausal breast cancer. J Gynecol Oncol. 22(2): 110-119.
- Wen C, Wu L, Fu L, Wang B, Zhou H (2017) Unifying mechanism in the initiation of breast cancer by metabolism of estrogen (Review). Mol Med Rep 16(2): 1001-1006.
-
Amani E Khalifa. Hypothyroidism, Hypertension and Breast Cancer: Scientific Insights. Adv Can Res & Clinical Imag. 1(1): 2018. ACRCI.MS.ID.000501.
-
Hypothyroidism, Thyrotropin Stimulating Hormone, Normal Free Thyroxine, Serum Lipoproteins, Hypertension, Prolactin, Estrogen, Norepinephrine, Polycyclic Aromatic Hydrocarbons, Estrogens, Catechol O Methyltransferase, Enzyme, Risk Factors, Peroxidases, Cancerous Estrogens, Metabolism, Hydroxylation, Polycyclic Aromatic Hydrocarbons, Androstenedione & Testosterone
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






