 Research Article
Research Article
A Comparative Study Between the Oncological Results of Right and Left Colic Cancers
M Abid*1, F Chaib-Eddour1, Y Bellah1, N Hammouda1, M Brahimi1, B Ouadfel1, Z Kordjani1, R Bouzouagh1, Z Benabdelhafidh1 and A Hammani2
1Department of Cancer Surgery, Anti-Cancer Center, Batna
2Department of Surgery, Pierre and Marie Curei Center, Alger
M Abid, Department of Cancer Surgery, Anti-Cancer Center, Batna.
Received Date: October 29, 2019; Published Date: November 07, 2019
Abstract
Introduction: There are embryonic, anatomical, and histological differences between right colon (RCC) and left colon (LCC) cancers. Many studies have sought to determine survival and prognosis based on the location of the tumor. The present study aims to compare the oncological results between RCC and LCC after curative resection.
Materials and methods: It is about a bi-centric retrospective study. The data collection of patients undergoing curative resection for RCC and LCC was made over a period, from July 2004 to December 2015.
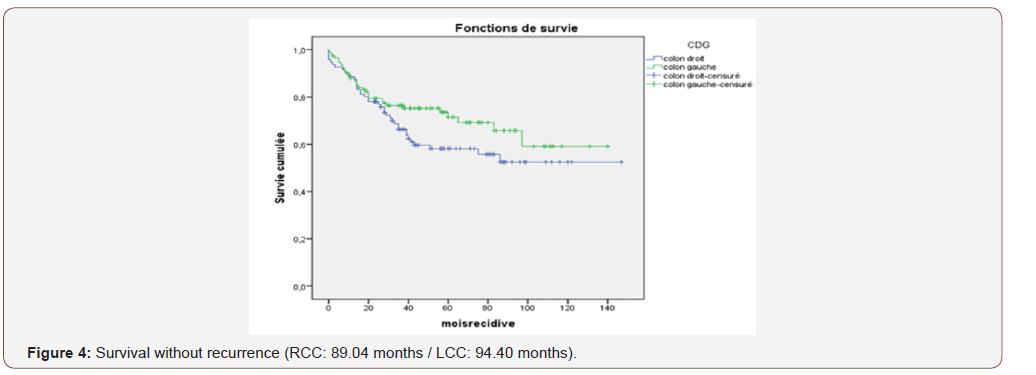
Results: On 353 patients, 156 were operated for RCC or 44% and 197 were operated for LCC or 56%. No significant difference in age or sex or time to surgery. It is noted that LCCs were diagnosed at a later stage. RCCs had more carcinoma type poorly differentiated. The median follow-up is 96 months. The survival curves are practically superimposable over a 36-month follow-up, as well as for survival without recurrence. Beyond and up to 96 months, the recurrence-free survival following a LCC is 94.40 months, higher than the one of the RCC which is 89.04 months. Overall survival is almost identical. LCCs have better survival for stage I cancers (P = 0.01).
Conclusion: On the basis of the current data, LCCs have a better survival result than RCC after curative resection (especially for stage I and SWR). Larger scale studies are needed. Determination of the impact on screening and appropriate specialized treatment related to the localization of 1 colon cancer is necessary.
Keywords: Colorectal cancer; Localization; Survival
Introduction
Colon cancer is the third most common cancer in the world, with an estimated incidence of 72,090 cases among men and 70,480 among women in the United States each year. It is also the third leading cause of cancer death with approximately 26,580 deaths of men and 24,790 deaths of women each year [1]. The gross incidence of colorectal cancer among Algerian men doubled between 2008 and 2015. In 2015, the incidence of colorectal cancer in men is getting closer and closer to that of the lung, and prostate cancer is returning to the third place before bladder cancer. Colorectal cancer is the second most common cancer in Algiers after breast cancer [2]. There are proofs that the right colon cancer (RCC) is different from left colon cancer (LCC) and rectal cancer. There are differences in embryological origins, as well as anatomical, histological, genetic and immunological differences between RCC and LCC [1,3]. The segments of the proximal and distal colon have different embryological origins. The first develops from the midgut and the last from the posterior intestine. Both are physiologically different [4]. The main treatment for colon cancer is surgical resection. The different types of resection depend on the location of the tumor are right hemicolectomy, enlarged right hemicolectomy, left hemicolectomy and left and right segmental colectomies. The same oncologic findings were obtained in the literature for both classical and laparoscopic approaches. RCCs are associated with iron deficiency anemia, late stage and advanced age. In addition, RCC has a tendency to involve large polypoid lesions, exophytic, developing in the lumen of the colon. In contrast, LCCs tend to include invasive constrictive lesions encircling the lumen and causing obstruction. [5] Tumor samples also showed differences in gene expression between RCC and LCC [4-7]. Most studies have reported poorer oncologic results in patients with RCC compared to patients with LCC. [8]. However, recent studies have indicated that the prognosis of localized RCCs is better than that of LCCs [9,10]. The influence of these potential differences on the prognosis has not been validated. Others concluded that there is no significant difference in overall survival (OS) between different tumor locations. [11,12]. The present study aims to compare the oncological results between RCC and LCC after curative resection.
Materials and Methods
Between July,2004 and December 2015, 353 patients underwent surgery for right and left colon adenocarcinoma, non-metastatic. Colon colonization, transverse colon cancer, recto-sigmoid hinge and rectal cancer were excluded. This is a bi-centric retrospective study whose data were collected prospectively. Patients were closely monitored and included in a database until December 2015 or until death. Patients in both groups were compared on the following parameters: age, sex, American Society of Anesthesiology (ASA) classification, presence of lympho-vascular invasion, degree of histological differentiation, mucinous histological subtype, number of lymph nodes removed, surgical margins, TNM classification (UICC, 7th edition), serum carcinoembryonic antigen (CEA) levels at the time of diagnosis, overall survival and no recurrence. The statistical analysis was done by SPSS software (Statistics 22). The Kaplan-Meier method was used to establish survival curves. The use of log rank tests, Cox model and Fisher’s exact test to analyze overall survival and recurrence-free survival and evaluate the significance.
These tests allowed to perform a univariate and multivariate analysis, to look for the association of independent variables with the recurrence of the tumor and the overall survival.
Result
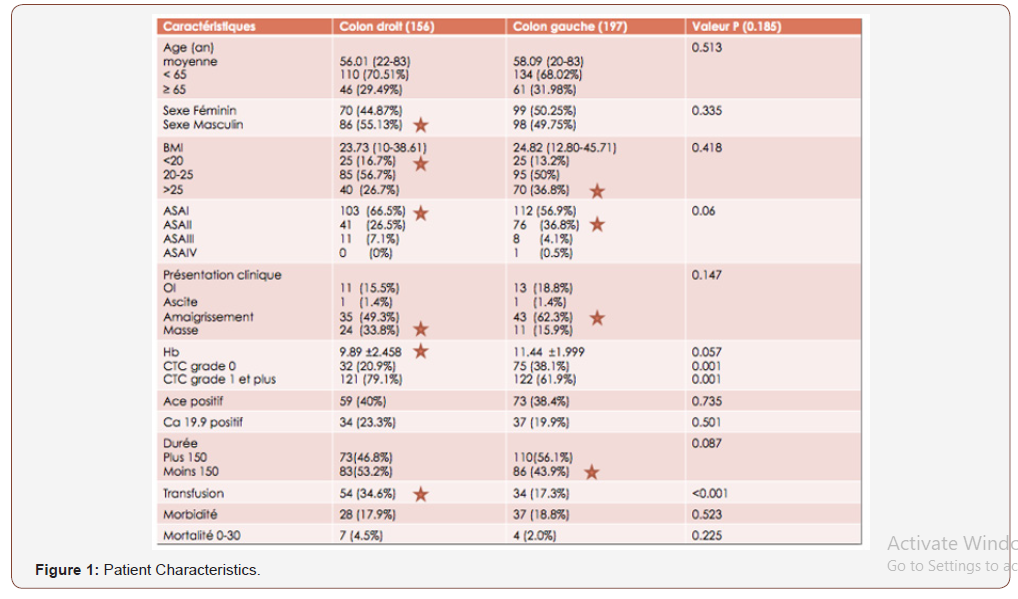
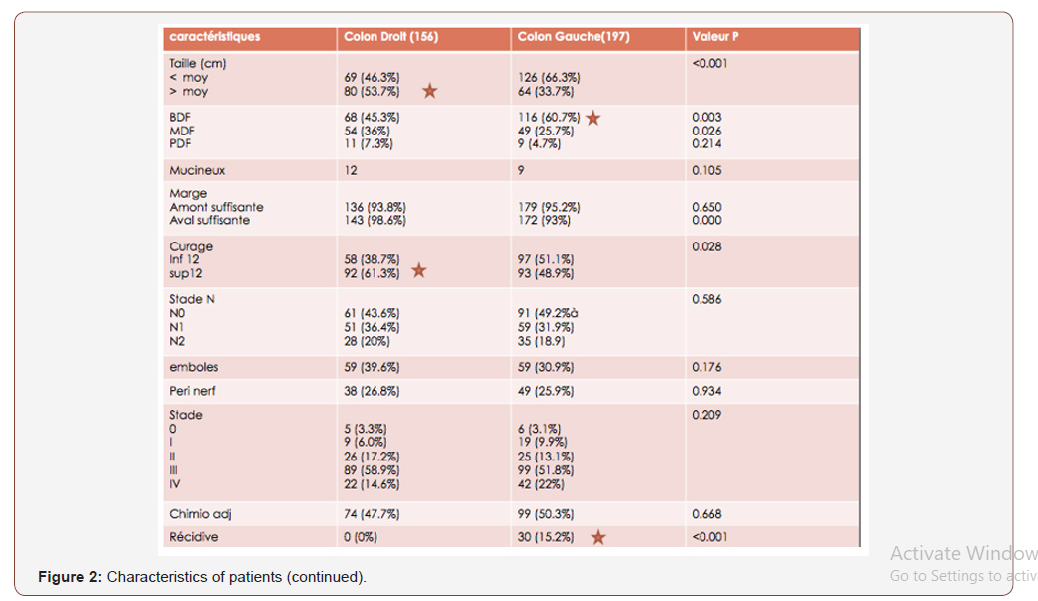
Among the 353 patients, 156 were operated for RCC or 44% and 197 were operated for LCC or 56%. Both groups were analyzed and compared. The average age was 56 (range: 22-83 years). Figures 1 and 2 summarize the clinical, histological and oncological findings of the subjects (Figures 1).
The median follow-up is 96 months. The survival curves are practically superimposable over a 36-month follow-up, as well as for survival without recurrence. Beyond and up to 96 months, the recurrence-free survival following an LCC is 94.40 months, higher than that of the RCC which is 89.04 months (Figure 2 and 3).
The overall survival is almost identical, it is 77.65 months for the RCC and 78.52 months for the LCC (Figure 4).
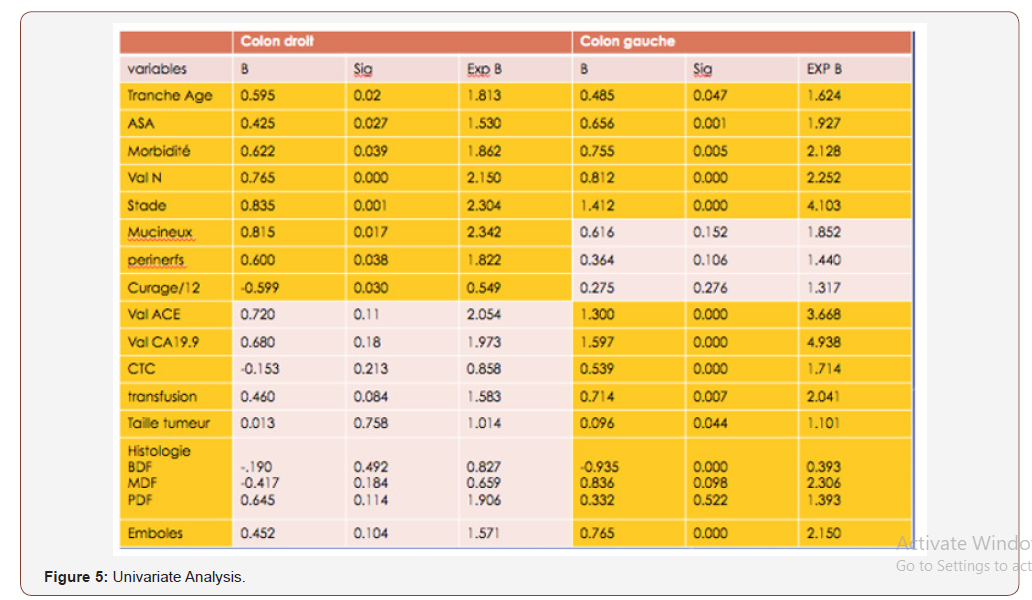
LCCs have better survival for stage I cancers (P = 0.01). No significant difference between the 2 groups for morbidity (17.9% vs 18.8%). The 30-day mortality is higher in the RCC group at 4.5% (7 patients) vs 2% (4 patients) in the LCC group. In univariate analysis, the poor prognostic factors for the overall survival rate that have been identified are advanced age (over 65 years), T3-4 stages, N stage (N1-2), and postoperative morbidity for both groups (Figure 5).
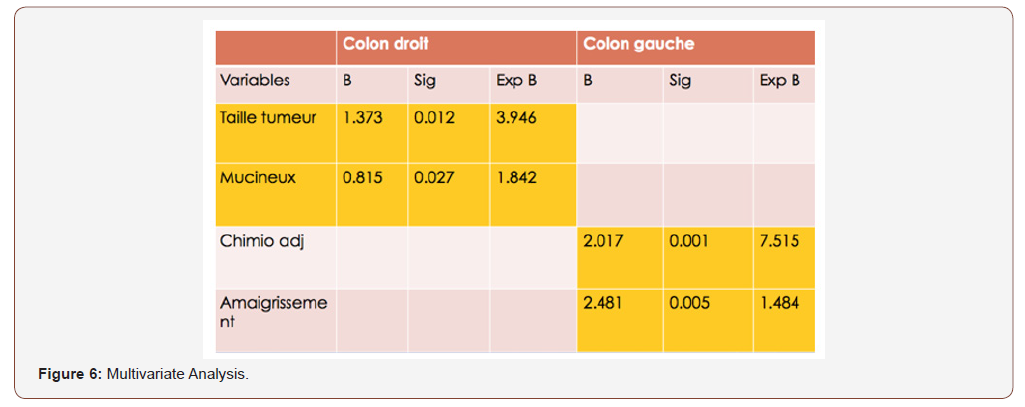
The number of lymph nodes removed, the peri-nerval girdling and the mucinous histological type for RCC. And the large size of the tumor, the ASA classification (II and III), high CEA and CA 19-9 levels, blood transfusion, the presence of vascular emboli and the degree of differentiation for the LCC group. In multivariate analysis, poor prognostic factors for overall survival rate are: Large tumor size and mucinous histological type for the RCC group. Weight loss and adjuvant chemotherapy for the LCC group (Figure 6).

Discussion
The present study aims to compare the oncological results between RCC and LCC after curative resection. Colon cancer has different clinical pathological features and genetic differences between the right colon and the left colon. Many studies have reported that patients with RCC are older and more often women; moreover, they have more advanced tumor stages, an increased tumor size, more often poorly differentiated tumors and different models of biological tumors [13,14]. The LCC incidence rate is higher than that of the RCC, and the latest figures released by the American Cancer Society confirm a higher proportion of LCC (51%) than the RCC (42%) in the United States [15]. In this study, the LCC (n = 197, 55.8%) is greater than the RCC (n = 156, 44%) over the same period (2004-2015).In this study, mean age and sex ratio are not significantly different between the 2 groups. The median ages are 56.01 years (RCC) and 58.09 years (LCC) (P=0.513) and the sex ratio is 0.81 (RCC) and 1.01 (GCC) (P = 0.335). Indeed, previous studies have reported poorer oncologic results in patients with RCC compared to patients with LCC [16-18]. The survival curves are practically superimposable on a 36-month follow-up (OS + SWR). Beyond and up to 96 months, the recurrence-free survival following an LCC is 94.40 months, higher than that of the RCC which is 89.04 months. Overall survival at age 8 is almost identical, at 77.65 months for RCCs and 78.52 months for LCCs. LCCs have better survival for stage I cancers (P = 0.01).
In univariate analysis, the poor prognostic factors for the rate of ILI that have been identified are advanced age (over 65 years), T3-4 stages, stage N (N1-2), and postoperative morbidity for 2 groups. The number of lymph nodes removed, the peri-nerval girdling and the mucinous histological type for RCC. And the large size of the tumor, the ASA classification (II and III), high CEA and CA 19-9 levels, blood transfusion, the presence of vascular emboli and the degree of differentiation for the LCC group. In the present study, the parameters that were analyzed as prognostic factors for OS and SWR after surgery in multivariate analysis were: The large tumor size and mucinous histological type for the RCC group, weight loss, and adjuvant chemotherapy for the LCC group. Indeed, the location of the tumor does not appear as an independent prognostic factor for OS after surgical treatment in colon cancer. The present study has several limitations, including that it is retrospective, selection bias and a small sample. Nevertheless, it showed results similar to those of previous studies [16-18].
Conclusion
RCCs are associated with a more advanced stage, a large tumor size, more often poorly differentiated (mucinous) tumors, more lymph nodes removed and more peri-nerve sheathing than LCCs in this study. present study. On the basis of current data, LCCs have a better survival result than RCC after curative resection (especially for stage I and SWR). In contrast, tumor localization does not appear to be an independent prognostic factor for OS after surgical treatment in colon cancer. Larger scale studies are needed. Determination of the impact on screening and appropriate specialized treatment related to the localization of colon cancer is necessary.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Jemal A, Bray F, Center M, Ferlay J (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Registre des Tumeurs d’Alger – Année (2015) Algiers Tumor Registry.
- Bufill JA (1990) Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 113: 779–788.
- Gervaz P, Bucher P, Morel P (2004) Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol 88: 261-266.
- Bale AL, Penney MD, Allison MC (2005) The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis 7: 398–
- Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, Laurberg S, Laiho P, et al. (2005) Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut 54: 374-384.
- Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, et al. (2003) Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev 12: 755-762.
- Jess P, Hansen IO, Gamborg M (2013) Danish Colorectal Cancer Group A nationwide Danish cohort study challenging the categorization into right-sided and left-sided colon cancer. BMJ Open 3: e002608.
- Warschkow R, Sulz MC, Marti L (2016) Better survival in right-sided versus left-sided stage I-III colon cancer patients. BMC Cancer 16: 554.
- Moritani K, Hasegawa H, Okabayashi K (2014) Difference in the recurrence rate between right- and left-sided colon cancer: a 17-year experience at a single institution. Surg Today 44: 1685–1691.
- Wiggers T, Arends JW, Volovics A (1988) Regression analysis of prognostic factors in colorectal cancer after curative resections. Dis Colon Rectum 31: 33-41.
- Steinberg SM, Barkin JS, Kaplan RS, Stablein DM (1986) Prognostic indicators of colon tumors. The Gastrointestinal Tumor Study Group experience. Cancer57: 1866-1870.
- Saltzstein SL, Behling CA (2007) Age and time as factors in the left to right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Ckin Gastroenterol 41: 173–177.
- Benedix F, Kube R, Meyer F (2010) Colon/Rectum Carcinomas (Primary Tumor) Study Group Comparison of 17,641 patients with right and left sided colon cancer: differences in epidermilogy, perioperative course, histology, and survival. Dis Colon Rectum 53: 57-64.
- Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics 2014. CA Cancer J Clin 64: 104-117.
- Hansen IO, Jess P (2012) Possible better long-term survival in left versus right- sided colon cancer: a systematic review. Dan Med J 59: 4444.
- Yahagi M, Okabayashi K, Hasegawa H (2016) The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg 20: 648-655.
- Petrelli F, Tomasello G, Borgonovo K (2016) Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol 27: 4227.
-
M Abid, F Chaib-Eddour, Y Bellah, N Hammouda, M Brahimi, et al A Comparative Study Between the Oncological Results of Right and Left Colic Cancers. Adv Can Res & Clinical Imag. 2(2): 2019. ACRCI.MS.ID..000535.
-
Colic Cancers, Bladder cancer, Tumor, Hemicolectomy, Carcinoembryonic antigen, Lymph nodes, Oncology, Prognostic factors
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






