 Research Article
Research Article
Prediction of Non-Sentinel Node Metastasis in Breast Cancer - A Population-Based Study
Eva Vikhe Patil1*, Ivan Shabo2,3, Oliver Gimm1, Lars-Gunnar Arnesson1 and Helena Fohlin4
1Department of Surgery and Department of Biomedical and Clinical Sciences, Linkö-ping University, SE-581 83 Linköping, Sweden
2Department of Molecular Medicine and Surgery, Karolinska Institute, Stockholm SE-171 77 Sweden
3Department of Breast Cancer, Sarcoma and Endocrine Tumors, Karolinska University Hospital, SE-171 76 Stockholm, Sweden
4Regional Cancer Center of Southeast Sweden and Department of Biomedical and Clinical Sciences, Linköping University, SE-581 83 Linköping, Sweden
Eva Vikhe Patil, Department of Surgery, University Hospital, Linköping, SE 581 85 Linköping, Sweden.
Received Date: February 21, 2022; Published Date: March 10, 2022
Abstract
Background
It is debated if all breast cancer (BC) patients with sentinel lymph node metastasis need axillary lymph node dissection (ALND). Based on clinical and biological factors, we explore a statistical model for predicting non sentinel node metastasis (non-SNm) in patients with positive SN (SN+).
Methods
We obtained data on patients from the Swedish National Quality Register for BC patients operated Jan 2008 - May 2012. Pearson´s chi-squared test was performed to compare clinical data with presence of non-SNm. The risk of non-SNm, estimated as odds ratio (OR), was calculated with multivariable logistic regression analysis and the results were visualized with a nomogram. Receiver operating characteristic (ROC) analysis was used to evaluate the discriminatory ability of the regression model (Prisk) in predicting non-SNm.
Results
Out of 5382 patients with SN+, 3181 had macro metastases at sentinel node biopsy and were treated with ALND. The non-SNm was statistically significant correlated to the proportion of SN+ (OR increased from 1.41 to 3.75 with raised proportion, p<0.001), tumor size (OR= 1.70, p<0.001), LVI-lymphovascular infiltration (OR= 1.63, p<0.001), HER2 expression (OR= 1.49, p=0.004) and multifocality (OR= 1.28, p=0.04). A cut-off value of 0.3 for Prisk based on the logistic regression model, yielded a sensitivity of 83.2% and specificity of 34.7 % in predicting non-SNm. The results were visualized with a nomogram where the proportion of SN+ was the most important factor.
Conclusion
In this study we present a statistical score encompassing BC biology with good sensitivity and acceptable specificity that may be used in predicting non-SNm as a complement to traditional staging system used in clinical assessment of BC.
Keywords: Breast cancer; Sentinel node; Axillary metastases; Nomogram/Scoring system
Introduction
Axillary lymph node metastasis from primary breast cancer (BC) is the earliest clinical presentation of metastatic BC indicating the presence of systemic disease and the need for oncologic treatment [1]. Axillary lymph node dissection (ALND) was mandatory in BC treatment for many years but during the last decades, sentinel lymph node biopsy (SNB) has been the routine technique for axillary staging in BC patients [2]. In cases with negative sentinel node (SN), the likelihood for further spread to the axillary non-SN lymph nodes is less than 5% [3-5]. Consequently, unnecessary ALND can be avoided in patients with negative SN, reducing the risk of ALND associated surgical morbidity [6]. Even with micrometastases, as shown in IBCSG 23-10 study, the number of axillary events is low [7]. In this regard, it is important to note that the AMAROS study showed that both ALND and axillary radiotherapy provided excellent and comparable locoregional control of selected SN+ patients [8].
Therefore, the trend for BC treatment is currently changing towards minimizing axillary surgery, despite the presence of metastatic SN [9-12]. In this study, including patients with positive sentinel-node (SN+), we aimed to identify clinical markers to predict the presence of non-SN metastasis (non-SNm). Identification of patients with SN+ having low risk for non-SNm is essential to avoid unnecessary ALND.
Methods
Patient material
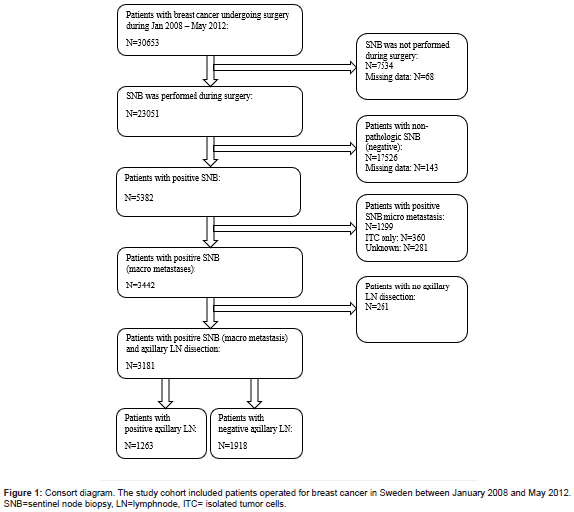
From the Swedish National Quality Registry for Breast Cancer (NKBC) we obtained data on patients (n=30653) treated with surgery for invasive or in situ BC in Sweden during January 2008 to May 2012. The study was approved by the NKBC register group and the Swedish Ethical Review Authority (Diarnr: 2020-06061). The NKBC, is a national database that has been running since 2008 and covering 99% of patients treated for BC in Sweden [13]. This register includes clinical variables such as age, sex, ICD, and Systematized Nomenclature of Medicine (SNOMED) codes, TNM classification, type of performed surgery, information about the SNB status, presence of non-SNm and tumor hormonal status. We excluded all patients with missing data about SNB, negative SN, and patients not treated with ALND. Out of 5382 patients with SN+, 3181 (59%) cases had positive macro metastases at SNB and were treated with ALND. Those cases constituted the patient cohort investigated in subsequent statistical analysis (Figure 1).
SNB procedure
The SNBs were identified in accordance with routine surgical procedures. Briefly, a radioactive isotope (Technetium) was injected adjacent to the primary BC, subcutaneously or intracutaneously. Preoperative lymphoscintigraphic images were often obtained for evaluating the position of the SN. At the time for surgery, Patent Blue® was injected with the same technique used for isotope injection. The SNB was detected with a handheld gamma probe and defined as the lymph nodes emitting radioactivity and/or expressing blue colour. In majority of the patients, peroperative histopathologic examination of SNB was performed by using frozen section. Axillary lymph node clearance was often performed directly if any metastasis was found at SNB.
The patients in this study were treated according to the BC treatment guidelines from European Society for Medical Oncology (ESMO) [14]. At the time of the study, axillary lymph node clearance was performed when macro- or/and micro metastasis was found at SNB, but not for patients with SN containing only isolated tumor cells (ITC). The number of total axillary nodes removed, SNs, positive SNs and all positive nodes was registered for each patient. Out of these data, the number and proportion rates of positive SN and non-SNm were calculated.
Statistical analysis
To explore the association between non-SNm and established clinical factors used in assessment of BC, all patients (n=3181) with macro metastasis in at least one lymph node of the SN(s) and followed by ALND were included in the statistical analyses. The analyses were performed with Pearson´s chi-squared test comparing clinical data with presence of non-SNm. Clinical variables with statistically significant correlation to the presence of non-SNm were included in a subsequent multivariable logistic regression analysis. For each patient, a risk value (Prisk) based on the formula from the logistic regression model was calculated (see supplement). The impact of each of the regression coefficients from the logistic regression model was illustrated graphically in a nomogram with the usercontributed program nomolog for Stata [15]. The nomogram provides a straightforward method of predicting and visualizing the probability of non-SNm by assigning a score proportional to the log of the odds ratio (OR) (i.e., estimated regression coefficients) to all variables included in the multivariable logistic regression model. The command nomolog was applied using default values. Receiver operating characteristic (ROC) curves were used to evaluate the discriminatory ability of the regression model in predicting non-SN metastases. The area under curve (AUC) was calculated with 95% CIs. The statistical analyses and graphics were performed with software IBM SPSS Statistics 27.0 [16] and Stata/SE 13.1 [17].
A P value of < 0.05 was considered statistically significant.
Results
In total, SNB was performed in 23051 patients and 5382 (23%) of them had positive SN. Micrometastases and ITC were found in 1299 (24%) and 360 (7%) patients with SN+, respectively. Macro metastasis in SNs were found in 3442 cases. Due to reasons not defined in the NKBC data base, 261 (8%) patients with SN+ did not undergo ALND. Axillary lymph node dissection was performed in 3181 (92%) cases and out of these 1263 (39.7%) patients had non- SNm (Figure 1).
The number of nodes in all SNB
The mean number of lymph nodes removed at SNB was 2 (range 1-9). The amounts of harvested nodes at SNB were 1 in 9274 (41%), 2 in 7169 (32%) and more than 2 in 6182 (27%) of the patients (missing data for 426 patients). The number of nodes removed during SNB was lower in older patients (p<0.001) and if the tumor was detected with mammography screening (p< 0.001) (Table 1).
Table 1:The number of SN removed at the time of surgery.
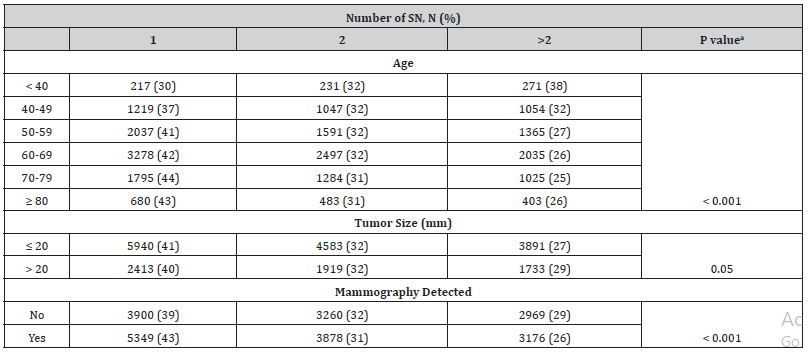
SN=sentinelnode aPearson’s chi-squared test
Clinical data associated with metastasis in non-SN lymph nodes
The number of lymph nodes with macro metastases removed at SNB was associated with presence of non-SNm. In 248 patients with more than 2 SN+ removed during SNB, non-SNm was detected in 157 cases (63%) (Table 2). The corresponding rates in patients with 1 and 2 SN+ were 34% and 50%, respectively (p<0.001). The presence of non-SNm was significantly associated with the proportion of pathologic SN detected at SNB. Non-SNm was found in 161 patients (26%) with SN proportion less than 0.5, in 345 patients (34%) with SN proportion between 0.5 and 0.99, in 436 patients (44%) with 1 of 1 SNs, in 206 patients (57%) with 2 of 2 SNs and in 111 patients (71%) with 3 of 3 SNs at SNB (p<0.001). Non-SNm was statistically significant more common in patients with tumors > 20 mm (47%) compared to those with tumors ≤ 20 mm (32%) (p<0.001). The non-SNm was more often found in patients with primary tumors expressing lymph vascular invasion (LVI) compared to those without LVI (51 % vs 35%, p< 0.001). The presence of non-SNm was associated with advanced tumor grade as it was more common in patients with NHG 3 (44%) and NHG 2 (40%) tumors compared to those with NHG 1 (32%) tumors (p<0.001).
Table 2:Presence of non-SN metastases related to biological and clinical data in patients with macro metastases in SN.
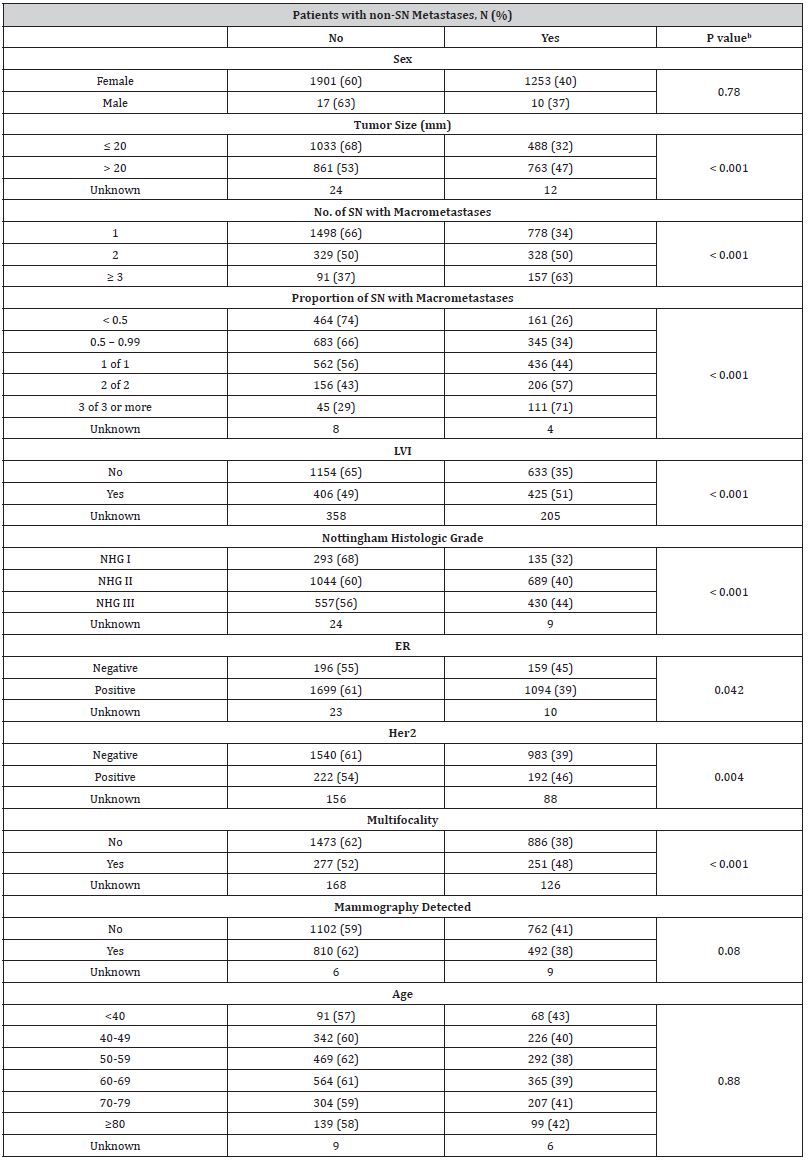
ER= Estrogen receptor; Her2= Human Epidermal Growth Factor Receptor 2; LVI= lymphovascular invasion; NHG= Nottingham Histologic Grade; SN= Sentinel node; bPearson’s chi-squared test
Metastasis in non-SN was also significantly associated to biological markers previously known to be related to BC dissemination. Out of 2793 patients with estrogen receptor (ER) positive tumors, non-SNm was detected in 1094 patients (39 %). In patients having ER negative tumors, non-SNm was found in 159 (45 %) patients (p= 0.042). The corresponding rates in patients with human epidermal growth factor receptor 2 (HER2) negative and positive tumors were 983 (39%) and 192 (46%), respectively (p=0.004). In case of multifocal tumors, non-SNm was found in 251 patients (48%), which was a significantly higher proportion compared to patients with solitary primary tumors (n=886, 38%) (p<0.001). No statistically significant association was found with age (p=0.88), sex (p=0.78) or mammography screening (p=0.08) as a diagnostic modality (Table 2).
Estimation of metastasis in non-SN lymph nodes
Estimated as odds ratio (OR), the risk of having non-SNm increased significantly in relation to the proportion and the number of nodes with macro metastasis found at SNB. If the number of SNs investigated was at least 2 and all nodes were pathologic, the OR for having non-SNm was 3.75 compared to the reference category (proportion of macro metastases among SN harvested < 0.5). If only one SN was investigated and if this one contained metastasis, the OR was 2.25 and if macro metastasis was found in at least half of the SNs, though not in all of them, the OR was 1.41 (Table 3).
Table 3:The logistic regression showing odds ratio for non-SN metastasesa.
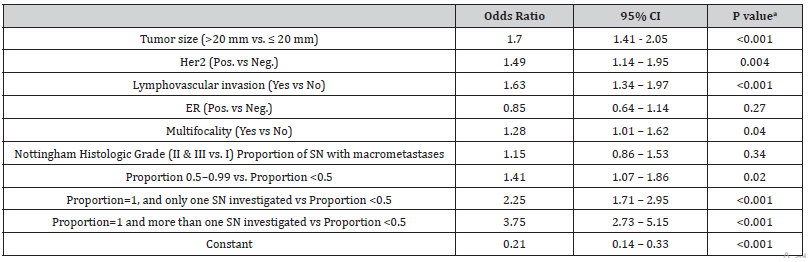
ER= Estrogen receptor; Her2= Human Epidermal Growth Factor Receptor 2; LVI= lymphovascular invasion; NHG= Nottingham Histologic Grade;
SN= Sentinel node
aThe logistic regression analysis was performed for patients where we had data on all variables included (2139 patients).
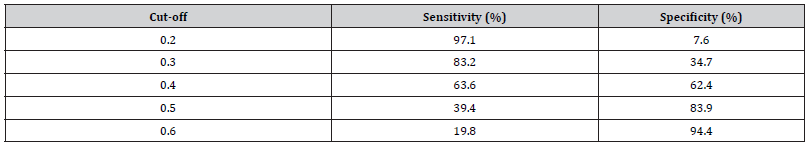
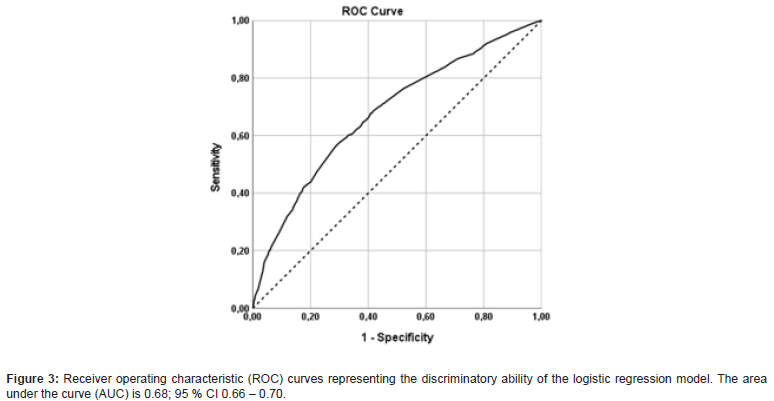
The risk of having non-SNm was significantly correlated to patients with tumors larger than 20 mm (OR 1.70, 95% CI 1.41-2.05 and p <0.001), positive HER2 expression (OR 1.49, 95% CI 1.14- 1.95, p = 0.004), multifocality (OR 1.28, 95% CI 1.01-1.62, p=0.04) and LVI (OR 1.63, 95% CI 1.34-1.97, p <0.001). The result from the logistic regression model was visualized in a nomogram showing the predicted probability of non-SNm for each patient (Figure 2). The higher score in the nomogram, the higher probability of non- SNm. For example, the total score of 6 gives a probability of less than 0.30 for non-SNm and the total score of 10 gives a probability of approximately 0.40. The ROC analysis based on the logistic regression model had an area under the curve (AUC) of 0.68; 95 % CI 0.66 – 0.70 (Figure 3). A cut-off of 0.3 for the Prisk value yielded high sensitivity (83.2%), but lower specificity (34.7 %). Nevertheless, a cut-off for the Prisk value of 0.2 yielded a sensitivity of 97.1% but a low specificity of 7.6%. The sensitivity and specificity rates related to other Prisk cut-off values are reported in Table 4.
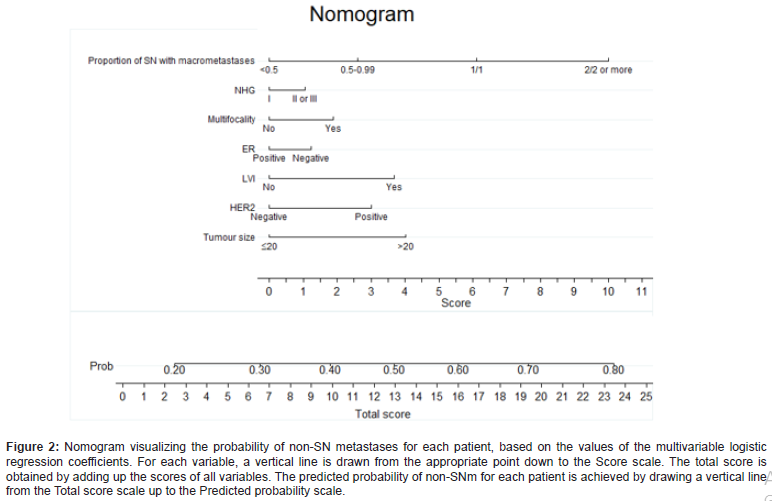
Table 4:The logistic regression showing odds ratio for non-SN metastasesa.
Discussion
In this study, we used pre-/postoperative clinical and biological tumor data to create a predictive model for selecting patients with macro metastases at SNB to perform ALND or not. The number and proportion of pathologic nodes at SNB are significantly related to non-SNm. The model of Prisk calculation, based on clinical and biological features of BC, provides a good estimation for the presence of non-SNm in patients with pathologic SN. Over the past decades, it has been empirically found that ALND might not be a necessary treatment in all patients with pathological SN, hence the current trend in BC treatment is changing towards minimizing axillary surgery [11,18,19]. Consistent with previous reports, our study shows that 60.3% of BC patients with pathologic SN do not have non-SNm and thus are not expected to benefit from subsequent ALND. Refraining ALND in some patients despite SN+ is still controversial [2,7,8] due to difficulties of selecting the cases that will benefit from this surgical procedure. As a first step, it is desirable to correctly select the majority of the 60% of patients without non-SNm to avoid complementary ALND.
The arguments against omission of ALND is that metastasis in the axilla is still a significant prognostic factor guiding the selection of adjuvant treatment and surgical locoregional control of BC [10, 20]. Hence, developing predictive models based on clinical and biological data to estimate the risk of non-SNm is still essential to avoid unnecessary ALND [21]. Today, most tumor characteristics can be evaluated at routine preoperative core biopsy and ultrasound investigation. At the time of primary surgery, the presence of macro metastasis in SNB can be detected by frozen section of SNB. Thus, already during the primary surgery, one can tentatively estimate the risk of the presence of non-SNm and make decision for ALND, based on data obtained from preoperative core biopsy and peroperative SNB diagnostics. In several trials, no difference in tumor recurrence and overall survival was found in patients with ITC or micro metastases and omitting ALND in these cases is generally accepted [9, 22]. Moreover, Ingvar C et al recently showed in a study of more than 1500 patients with small breast cancers that omitting all axillary surgery was associated with very low rates of axillary events [23]. The presence of non-SNm has been shown to be related to the number of pathologic SNs, lobular phenotype and multifocal primary tumors [24].
In our study, tumor size, the proportion and number of positive nodes at SNB, LVI, multifocality and HER2 expression were associated with the presence of non-SNm. We combined tumor biological features and SNB characteristics in a statistical model to estimate the risk for the presence of non-SNm, attempting to improve the selection of patients with SN+ that will benefit from ALND. Based on this model, a Prisk of 0.4 yielded a sensitivity of 63.6% and specificity of 62.4% in predicting axillary non-SNm. This means that out of the patients where non-SNm were identified, 64% were correctly predicted to have non-SNm according to the model. A Prisk of 0.5 increased the specificity to 83.9%, albeit at the cost of the sensitivity 39.4 %.
The Tenon score, used in several studies for predicting axillary metastasis, has a sensitivity of 92.1% and specificity of 70.1% [25]. The Tenon score is based on 3 variables: presence of macro metastasis, tumor size and the proportion of positive nodes at SNB. It does not include tumor biological factors, such as LVI and HER2 expression [25,26]. Similar factors in Tenon scores are used in Memorial Sloan Kettering Cancer Center (MSKCC) nomogram [27,28]. In general, all models used to predict non-SNm in BC show lower accuracy when applied outside the populations they are developed in [29]. In most studies the inclusion criteria are 2 SN+ to refrain from ALND [8,18]. In our study, 50% of the patients with 2 SN+ actually had non-SNm. The predicted risk for non-SNm was increased by combining some risk factors in the nomogram. For example, 2 SN+ of 2 LNs investigated, LVI and tumor size >20 mm yields a total score of 18, which corresponds to a predicted probability of approximately 67%. Today, even with a limited spread in the axilla (1-2 positive SN) it is suggested to avoid ALND and the morbidity associated with this type of surgery. For example, in Sweden all eligible patients with up to 2 SN+ are currently offered to participate in the randomized clinical study, SENOMAC [12]. For those who are not included, ALND is recommended according to the Swedish national guidelines for BC [30]. Notably, this study does not take in consideration the biological properties of the primary tumor or the proportion of pathologic nodes in SNB.
The adjuvant oncologic treatment is assumed to eliminate residual disease after surgery. Hence, if we only perform diagnostic SNB-surgery, patients with metastasis in the SN should be offered adjuvant oncological treatment. In the clinical trial Z0011 [31] it was shown that the tangential radiation and systemic therapy was quite effective in controlling residual axillary node disease in patients that were not treated by ALND. Similar results were shown in the AMAROS study [8] that also had low arm morbidity in the radiotherapy group. Moreover, the ACOSOG Z0011 [31] and IBCSG 23-01 [7] trials showed that patients with 1-2 SN+ who were treated with breast conserving surgery with whole-breast irradiation and adjuvant systemic treatment, could be spared ALND without compromising locoregional control of BC or survival outcome. In light of these observations, the findings presented in our study indicate that patients with low number and proportion of pathologic SN, ER+ and HER2+ small tumors (≤ 20 mm) without LVI had significant low risk of having non-SNm and thus could be refrained from axillary treatment. The strength of our study is that it is population based and consist of large patient cohort. The scoring model is based on a combination of clinical and tumor biological factors, which tentatively might better encompass the malignant behaviour of the tumor. Moreover, the predictive model presented here can be applied in adjacent to primary surgery as a complement to traditional staging system used in clinical assessment of BC. Further studies are needed to validate this model in another data set and test it in relation to other clinical data, such as tumor specific survival and outcomes of adjuvant oncologic treatment.
Conclusion
There is a need for a predictive model based on tumor burden and biology to predict metastasis in axillary non-SN. In this study, we present a statistical score encompassing BC biology with good sensitivity and acceptable specificity that can be used in predicting non-SNm as a complement to traditional staging system used in clinical assessment of BC. We suggest that such a scoring model might be applicable in adjacent to primary surgery in order to avoid unnecessary ALND without compromising the oncological outcomes of BC surgery.
Acknowledgement
We would like to thank the NKBC Register Group (The Swedish National Quality Register for Breast Cancer) which approved the study and provided data.
Conflict of Interest
No conflict of interest.
References
- Balic M, Christoph T, Rachel W, Michael G, Nadia H (2019) St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 14(2): 103-110.
- Rutgers EJ, Jansen L, Nieweg OE, J de Vries, H Schraffordt Koops, et al. (1998) Technique of sentinel node biopsy in breast cancer. Eur J Surg Oncol 24(4): 316-319.
- Moo TA, Rachel S, Chau D, Monica M (2018) Overview of Breast Cancer Therapy. PET Clin 13(3): 339-354.
- Krag DN, Stewart JA, Thomas BJ, Ann MB, Seth PH, et al. (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(10): 927-933.
- Krag D, D Weaver, T Ashikaga, F Moffat, VS Klimberg et al. (1998) The sentinel node in breast cancer--a multicenter validation study. N Engl J Med 339(14): 941-946.
- Bergkvist L, Frisell J (2005) Multicentre validation study of sentinel node biopsy for staging in breast cancer. BJS (British Journal of Surgery), 92(10): 1221-1224.
- Galimberti V, Bernard FC, Giuseppe V, Paolo V, Elisa V, et al. (2018) Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol 19(10): 1385-1393.
- Donker M, Geertjan van T, Marieke S, Philip M, Cornelis J H van de Velde, et al. (2014) Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15(12): 1303-1310.
- Ozcan LC, Giuliano AE (2017) Is Axillary Lymph Node Dissection Necessary After a Positive Sentinel Lymph Node Biopsy? Adv Surg 51(1): 165-178.
- Bundred NJ, Nicola LPB, Emiel R, Mila D (2015) Is axillary lymph node clearance required in node-positive breast cancer? Nat Rev Clin Oncol,12(1): 55-61.
- Veronesi U, G Paganelli, V Galimberti, G Viale, S Zurrida, et al. (1997) Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349(9069): 1864-1867.
- de Boniface J, Jan F, Yvette A, Leif B, Johan A, et al., (2017) Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer 17(1): 379.
- Lofgren L, Sandra E, Kamilla K, Annette A, Charlotta L, et al. (2019) Validation of data quality in the Swedish National Register for Breast Cancer. BMC Public Health 19(1): 495.
- Aebi S, T Davidson, G Gruber, M Castiglione (2010) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 21 Suppl 5: v9-14.
- Zlotnik A, V Abraira (2015) A General-purpose Nomogram Generator for Predictive Logistic Regression Models. The Stata Journal 15(2): 537-546.
- IBM Corp (2020) Released 2020. IBM SPSS Statistics for Windows, V.A., NY: IBM Corp SPSS.
- StataCorp (2013) Stata Statistical Software: Release 13. College Station, T.S.L. STATA.
- Giuliano AE, Karla B, Linda McC, Peter DB, Meghan B Brennan, et al. (2010) ACOSOG Z0011: A randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node. Journal of Clinical Oncology 28(18_suppl): CRA506-CRA506.
- Giuliano AE, Karla VB, Linda McC, Peter DB, Meghan B Brennan, et al. (2017) Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 318(10): 918-926.
- Tallet A, Eric L, Monique C, Mathieu M, Marie B, et al. (2016) Locoregional treatment of early breast cancer with isolated tumor cells or micrometastases on sentinel lymph node biopsy. World journal of clinical oncology 7(2): 243-252.
- Dihge L, Bendahl PO, Rydén L (2017) Nomograms for preoperative prediction of axillary nodal status in breast cancer. British Journal of Surgery 104(11): 1494-1505.
- Yegiyants S, Lina MR, Philip IH, DiFronzo LA (2010) Completion axillary lymph node dissection not required for regional control in patients with breast cancer who have micrometastases in a sentinel node. Arch Surg 145(6): 564-569.
- Ingvar C, Ahlgren J, Emdin S, Lofgren L, Nordander M, et al. (2020) Long-term outcome of pT1a-b, cN0 breast cancer without axillary dissection or staging: a prospective observational study of 1543 women. Br J Surg 107(10): 1299-1306.
- Majid S, L Rydén, J Manjer (2019) Determinants for non-sentinel node metastases in primary invasive breast cancer: a population-based cohort study of 602 consecutive patients with sentinel node metastases. BMC Cancer 19(1): 626.
- Coutant C, Roman R, Eric F, Frederic M, François G, et al. (2009) Validation of the Tenon breast cancer score for predicting non-sentinel lymph node status in breast cancer patients with sentinel lymph node metastasis: a prospective multicenter study. Breast Cancer Res Treat 113(3): 537-43.
- Barco I, A García-Fernández, C Chabrera, M Fraile, E Vallejo, et al. (2016) The appropriate axillary procedure after a positive sentinel node in breast cancer patients: the "Hôpital Tenon" score revisited. A two-institution study. Clin Transl Oncol 18(11): 1098-1105.
- Houpu Y, Xie F, Yang Y, Tong F, Liu Peng, et al. (2019) Use of Memorial Sloan Kettering Cancer Center nomogram to guide intraoperative sentinel lymph node frozen sections in patients with early breast cancer. J Surg Oncol 120(4): 587-592.
- Kuo YL, Wen-Chung C, Wei-Jen Y, Lili C, Hui-Ping H, et al. (2013) Validation of Memorial Sloan-Kettering Cancer Center nomogram for prediction of non-sentinel lymph node metastasis in sentinel lymph node positive breast cancer patients an international comparison. Int J Surg 11(7): 538-543.
- Yildiz R, Murat U, Oğuz Hancerliogulları, Zafer Kılbaş, Erkan Ozturk, et al. (2015) Comparison of five different popular scoring systems to predict nonsentinel lymph node status in patients with metastatic sentinel lymph nodes: a tertiary care center experience. Springerplus 4: 651.
- Vårdprogram(2020)
https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/ - Caudle AS, Kelly KH, Susan LT, Karen H, Sarah MG, et al. (2012) American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol 19(10): 3144-3151.
-
Eva Vikhe Patil, Ivan Shabo, Oliver Gimm, Lars-Gunnar Arnesson, Helena Fohlin. Prediction of Non-Sentinel Node Metastasis in Breast Cancer - A Population-Based Study. Adv Can Res & Clinical Imag. 3(3): 2022. ACRCI.MS.ID.000565.
-
Breast cancer, Sentinel node, Axillary metastases, Nomogram/Scoring system
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






