 Case Report
Case Report
PDL-1 Negative Metastatic Bladder Cancer with Dramatic Response to Sixth-Line Nivolumab Therapy
Ali Kaan Güren*, Erkam Kocaaslan, Nargiz Majidova, Nadiye Sever, Pınar Erel, Yeşim Ağyol, Abdussamet Çelebi, Rukiye Arıkan, Selver Işık, İbrahim Vedat Bayoğlu, Osman Köstek and Murat Sarı
Division of Medical Oncology, Department of Internal Medicine, Marmara University School of Medicine, Istanbul, Turkey
Ali Kaan Güren Marmara University School of Medicine, Pendik Hospital, Department of Internal Medicine, Division of Medical Oncology, Pendik/ Istanbul, Turkey.
Received Date:January 07, 2025; Published Date:January 22, 2025
Abstract
Bladder cancer, 25-30% of patients presenting the metastatic stage at or after diagnosis. In addition to platinum-based chemotherapies, immune check point inhibitors and antibody drug conjugates are also used in the treatment. A 54-year-old male patient received multiple lines of chemotherapy for metastatic bladder cancer. Despite all treatments, progression of lesions was observed. Finally, the programmed cell death 1 (PDL1) negative patient was treated with nivolumab and a dramatic response was obtained. The patient was followed up with nivolumab treatment for 10 months without progression and developed immunotherapy-associated colitis in the 10th month. In this process, treatment was discontinued and the patient’s clinical progression. With nivolumab, we observed a dramatic disease regression and a 10-month progression-free survival in our metastatic bladder cancer. This emphasised the crucial role of immunotherapy in bladder cancer, even with a negative PDL-1 score.
Keywords: Nivolumab; Metastatic bladder cancer; Immunotherapy; beyond progression
Introduction
Bladder cancer is the 10th most common cancer in the world among all cancers. Although it is more common in men than women, the most common subtype (approximately 90%) is urothelial carcinoma. At the time of diagnosis, 75% of bladder cancers are non-muscle invasive bladder cancer (NMIBC) and 25% are muscle invasive bladder cancer (MIBC). 25-30% of all bladder cancers are metastatic at or after diagnosis [1, 2].
Platinum-based chemotherapy combinations have long been used in the treatment of bladder cancer in neoadjuvant, adjuvant and metastatic settings [3]. With the use of immunotherapy and antibody drug conjugates (ADC), an increase in expected survival times has been observed [4]. Nivolumab is an anti-programmed cell death 1 (PD-1) immuncheck point inhibitor used in the treatment of various types of cancer. In metastatic bladder cancer, it improves progression-free survival (PFS) and overall survival (OS), especially in programmed death-ligand 1 (PDL-1) positive groups [5]. We will report a case of PDL-1-negative MBC resistant to multiple series of chemotherapy with a dramatic favourable response to nivolumab treatment in the 6th line.
Case Report
A 54-years-old male patient. He had a history of diabetes mellitus and was on sitagliptin and metformin treatment. He had no history of smoking and alcohol consumption. In 2011, the patient was admitted to the hospital with painless haematuria and a malignant mass in the bladder was detected in the radiological examination. Radical cystoprostatectomy was operated. Pathology was reported as low grade urothelial carcinoma. Followed in remission until 2020 without adjuvant treatment.
In 2020, the patient was admitted to the hospital with painless haematuria and magnetic resonance imaging (MRI) detected a lesion compatible with recurrence at the junction of the prostatic urethra and penile urethra. The biopsy revealed high grade urothelial carcinoma and urethrectomy was performed in the patient who had no distant metastasis. Informed consent was obtained from the patient. Cisplatin (40mg/m2) treatment with radiotherapy was scheduled for the patient due to positive surgical margin. After 3 months, imaging performed showed that the involvement in the urethral region of the patient’s urethra continued to increase. At the same time, new lesions compatible with metastasis in bilateral lungs and a soft tissue mass destructing the sacrococcygeal bone were detected. Cisplatin (day 1, 80mg/m2) and gemcitabine (days 1-8, 100mg/m2 every 21 days) combination chemotherapy and radiotherapy was applied to the lesion in the sacrococcygeal area. After 4 cycles, progression was detected in the lung lesions and perineal lesion. The patient received 12 courses of weekly paclitaxel (80mg/m2) in 2nd line, 4 courses of adriamycin (60mg/m2 21 days 1) in 3rd line, 4 courses of pemetrexed (500mg/m2 21 days 1) in 4th line and 4 courses of vinflunine (320mg/m2 21 days 1) in 5th line. Progression was detected in lung lesions, sacrococcygeal and perineal lesions in control imaging performed at the 3rd month of all treatments. PD-L1 score determined by Ventana Bench Mark Ultra (SP263) was found to be 0%. Positron emission tomographycomputed tomography (PET/CT) performed after vinflunine treatment revealed 68x56 mm metastatic lesions in bilateral lungs, 62x47mm mass lesion in the perineal region, and 86x79 mm metastatic lesion destructing the bone in the sacrococcygeal region (Figure 1).
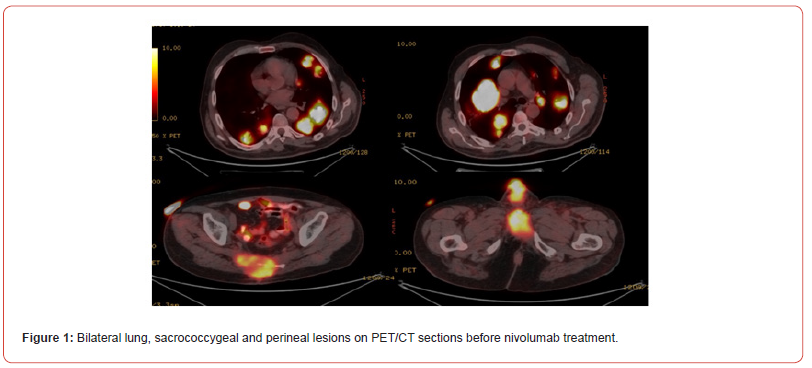
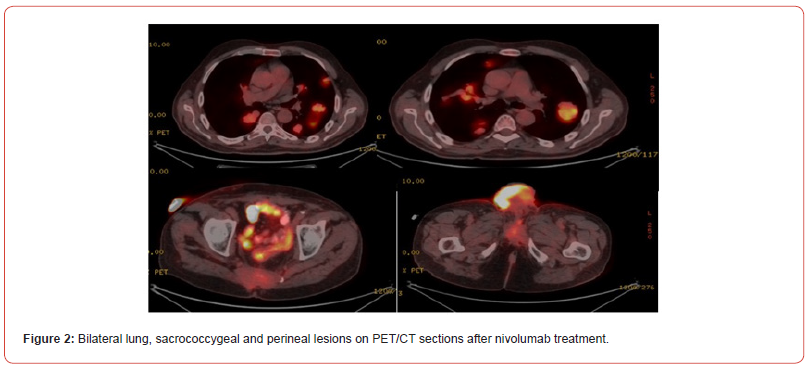
Single agent nivolumab (3mg/kg every 14 days) treatment was planned. After 6 courses of treatment, a dramatic favourable response was obtained on PET/CT (Figure 2). Nivolumab treatment was continued for 10 months (19 sessions in total). In the 10th month of treatment, the patient developed diarrhoea 15 times a day. Diarrhoea was bloodless, mucus-free and non-inflammatory. Direct examination was negative, stool culture was negative, Clostridium difficile, shigella and salmonella antigens were negative. Colonoscopy revealed mild recitis and normal colon mucosa. Rectal multiple biopsy pathology was reported as mild lymphoplasmacytic cell increase. Methyl prednisolone was started with a dose of 1mg/ kg (70mg) with the diagnosis of immunotherapy-associated colitis. The patient’s diarrhoea regressed with methyl prednisolone. The dose was decreased and methyl prednisolone was planned to be discontinued. During this period, clinical progression of lesions in the perineal and sacrococcygeal regions was observed. The patient, whose weight loss increased with diarrhoea and general condition deteriorated, died 2 months after the last nivolumab treatment and 12 months after the first treatment when a new treatment was planned, although prednisolone treatment regressed diarrhoea.
Discussion
In our patient with metastatic bladder cancer (MBC), who had a very high tumour burden and a negative pdl-1 score, we achieved a dramatic regression of the disease and a total progression-free survival of 10 months with nivolumab treatment after 5 series of conventional chemotherapy, all of which resulted in progression. This showed us the importance of the immunotherapy option in bladder cancer, especially in non-chemosensitive patients, even if the pdl-1 score is negative.
With the strong results of the EV302/Keynote-A39 study, the combination of enforumab vedotin-pembrolizumab in the first-line setting has forefront [4]. Also in the JAVELIN Bladder 100 study, similar survival results were obtained in patients diagnosed with MBC with positive results of maintenance Avelumab treatment after cisplatin-gemcitabine treatment [6]. At the time when our case was metastatic, platinum-based chemotherapies were still the standard of care and our case was treated in this setting. Due to social and financial reasons, immunotherapy was preferred at a later stage than it should have been.
Results were obtained in a phase 2 study on the use of Nivolumab treatment in the second and subsequent steps in patients with MBC. In this study, patients who had previously received platinum treatment were included and median overall survival (OS) was found to be 8.74 months. When OS was analysed according to PDL scores, median OS was 11.3 months in PDL-1>1% group and median OS was 5.95 months in PDL-1<1% group [7]. Although there is no phase 3 study on the administration of nivolumab as a single agent, we achieved an OS of 12 months in our case.
In MBC, nivolumab treatment provides a very favorable response in immunogenic tumours. Although markers such as tumour cells score (TC), Immune cells area score (IC), Immune cell present (ICP) are guiding in analysing the character of the tumour, their specificity and sensitivity are controversial [8]. In non-chemosensitive tumours, immunotherapy should not be ruled out even if immune markers are negative. While the PFS of 5 series of chemotherapy regimens was 3 months, the PFS of nivolumab treatment was found to be 10 months in the 6th series with the highest tumour burden.
Immunotherapies may induce many autoimmune side effects such as pneumonitis, thyroiditis, hypophysitis and colitis. immunotherapy-associated colitis is observed in 2% of patients and most commonly occurs in the first 6-8 weeks of treatment. In our case, grade 3 colitis was observed earlier than expected in the 10th month [9].
Although ADC-immunotherapy, immunotherapyimmunotherapy and chemotherapy-immunotherapy maintenance immunotherapy combinations have come to the forefront in MBC patients with recent studies, in real life, in cases where these drugs cannot be administered in our daily practice due to social and financial reasons, it demonstrates that single agent immune chechpoint inhibitors should be considered in the second series and beyond in patients who have not received immunotherapy before.
Funding
This research received no external funding.
Acknowledgment
None.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rabbani F, Perrotti M, Russo P, Herr HW (2001) Upper-tract tumors after an initial diagnosis of bladder cancer: argument for long-term surveillance. J Clin Oncol 19(1): 94-100.
- Patel VG, Oh WK, Galsky MD (2020) Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin 70(5): 404-423.
- Yin M, Joshi M, Meijer RP, Glantz M, Holder S, et al. (2016) Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 21(6): 708-715.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, et al. (2024) EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med 390(10): 875-888.
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, et al. (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18(3): 312-322.
- Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, et al. (2023) Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results from the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol 41(19): 3486-3492.
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, et al. (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18(3): 312-322.
- Martinez-Morilla S, Moutafi M, Rimm DL (2022) Standardization of PD-L1 immunohistochemistry. Mod Pathol 35(3): 294-295.
- Nielsen DL, Juhl CB, Chen IM, Kellermann L, Nielsen OH (2022) Immune checkpoint Inhibitor-Induced diarrhea and Colitis: Incidence and Management. A systematic review and Meta-analysis. Cancer Treat Rev 109: 102440.
-
Ali Kaan Güren*, Erkam Kocaaslan, Nargiz Majidova, Nadiye Sever, Pınar Erel et.all. PDL-1 Negative Metastatic Bladder Cancer with Dramatic Response to Sixth-Line Nivolumab Therapy. Adv Can Res & Clinical Imag. 4(5): 2025. ACRCI.MS.ID.000596.
-
Metastatic bladder cancer, Nivolumab treatment, Dramatic disease, Immunotherapy, Radical cystoprostatectomy, Radiotherapy, Diarrhoea, Immunogenic tumours, Chemotherapy, Diabetes mellitus
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






