 Research Article
Research Article
The Association of ABO Blood Types with Childhood Leukemia
Monya Abdullah Yahya El-Zine1, Maged Ali Amer Ali2 and Hassan Abdulwahab Al-Shamahy3*
1Department of Histopathology, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen
2Department of Neurosurgery, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen
3Medical Microbiology and Clinical Immunology Department, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen
Prof. Hassan A. Al-Shamahy, Faculty of Medicine and Heath Sciences, Sana’a University, P.O. Box 775 Sana’a, Yemen.
Received Date: August 23, 2023; Published Date: September 07, 2023
Abstract
Background and aims: The ABO antigens of the human blood group show diverse phenotypes, and the genetically generated glycoconjugate structures on the surface of red blood cells are crucial to both normal and pathological cell function. Since the discovery that antibodies and antigens are inherited, researchers have been examining the relationship between blood type and disease. The link between the ABO blood group and susceptibility to specific infectious and noninfectious disorders has, however, given rise to some problematic concerns due to the absence of antigens for some blood groups. Our study’s objective is to identify the frequency of various hematological malignancies associated with the ABO blood group in the pediatric cancer units at Al-Kuwait Hospital in Sana’a City.
Patients and method: Children with leukemia who received selected care at the Pediatric Leukemia Unit of Kuwait University Hospital in Sana’a were the subject of a cross-sectional investigation. Over a 5-year period, pediatric leukemia units developed group diagnoses and histological diagnoses in accordance with the French, American, and British classifications of pediatric leukemia. Age, sex, and blood types were investigated as leukemia-related factors. Rates and the computation of OR, CI, X2, and p values through probability tables (2x2 tables) were used to determine the relationship between leukemia and blood group in comparison to age- and sex-matched controls from the general population.
Results: Leukemia in 332 patients was identified, treated, and monitored. Leukemia was associated with mean SD age of 7.96 3.93 years by 6-10 years (67.8%). While there was no significant association with other blood groups, there was a significant association between gp AB and the risk of pediatric leukemia, with odds ratios of 2.3, 95% CI = 1.2-6.95, and p-value 0.01. Contrary to Rh-negative, which was a very significant risk factor for children leukemia with an associated odds ratio of 6.43, a CI of 2.2-6.3, and a p-value of 0.0001, however, Rh-positive was the protective factor against childhood leukemia.
Conclusion: Our ABO phenotypic research demonstrated a comparable relationship between Yemeni childhood leukemia risk and genetically determined human ABO blood types as AB blood group and negative Rh factor. However, further research is necessary, particularly at the molecular level in relation to ABO blood types and their correlation with leukemia.
Keywords: Childhood leukemia, Associated factors, Odds ratio (OR), ABO blood group, Rhesus factor, Yemen.
Introduction
On the surface of red blood cells, human ABO blood type antigens display diverse phenotypes and genetically produced glycoconjugate structures that are actively involved in the physiology and pathophysiology of the cells [1, 2]. Additionally, the blood type was determined by oligosaccharide structures unique to the antigens. Thus, whereas different glycosyl-transferase enzymes that aid in the attachment of sugar molecules to the oligosaccharide chain are primary gene products, so blood group antigens are secondary gene products. Others’ immune systems recognize these carbohydrate components as foreign substances and develop antibodies against them [3]. The ABO blood type system has been pivotal in numerous disease research from a medical perspective [4]. However, there have been some disputed concerns regarding the relationship between the ABO blood group and vulnerability to certain infectious and noninfectious disorders like malignancies and autoimmune diseases [5]. This is because some blood groups do not have antigens. Blood membrane form and function are altered in some blood types depending on antigen presence or absence. Functions that depend on the structure of blood types can link blood groups to both health and sickness [1, 6]. Blood group antigens can also be found on leukocytes, specific organs, plasma proteins, platelets, and a number of cell surface enzymes in addition to RBCs [7].
Body fluids such perspiration, saliva, breast milk, seminal fluid, urine, gastric secretions, and amniotic fluid can also include blood group antigens in soluble form [8, 9]. However, because most antigens are the result of a single gene, genetic changes like deletions, inversions, insertions, alternative splicing, or single-nucleotide polymorphisms (SNPs) can result in the emergence of brand-new antigens or even the complete loss of expression [3]. The ABO blood group antigens were the first to be identified and are arguably the most significant and extensively studied [10]. The RBCs’ glycoconjugate structures serve a variety of purposes, such as acting as transporters, channels, structural proteins, adhesion molecules, enzymes, and receptors for foreign ligands like viruses, bacteria, and parasites [11]. However, the precise mechanisms underlying the relationships between blood group antigens and illness in adhesion molecules are yet unknown [5].
Numerous research on the potential connection between blood types and particular diseases have been conducted since the 1953 discovery of a correlation between stomach cancer and blood type A [12]. Individuals can be divided into the four blood types (A, B, O, and AB) using traditional serological investigations [13, 14]. The ABO types are primarily determined by two antigens and two antibodies. Most of the time, a person’s type is determined by a particular mix of these four factors [14]. It could be used as an epidemiological marker or as a primary screening tool to identify high-risk populations if the risk of a number of different diseases are recognized for various ABO blood types [15]. Hence, the distribution of ABO and rhesus factor blood groups among patients with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) was investigated in this study.
Patients, Controls and Methods
The pediatric leukemia units of Al-Kuwait University Hospital in Sana’a were the subject of a cross-sectional study on children with leukemia who were given selected treatment. The French, American, and British classifications of pediatric leukemia were used to provide group diagnostics and histological diagnoses. Ages, sex, clinical symptoms, blood types, and Rhesus factors were explored as factors linked to developing leukemia. 300 healthy children were randomly chosen from the community to serve as the study’s control group. The control group and the study’s 332 leukemia patients were age- and sex-matched.
Statistical analysis
The data analysis was done using the Epi Info statistical tool, version 6 (CDC, Atlanta, USA). when the data was regularly distributed, expressing the quantitative data as mean values and standard deviation (SD). Chi square test was used to compare two variables, expressing the qualitative data as percentages, and calculating the P value. When comparing leukemia to healthy controls, the odds ratio (OR) was utilized to identify the blood types and Rhesus factor that were related with the disease. Statistics were considered significant if P < 0.05.
Results
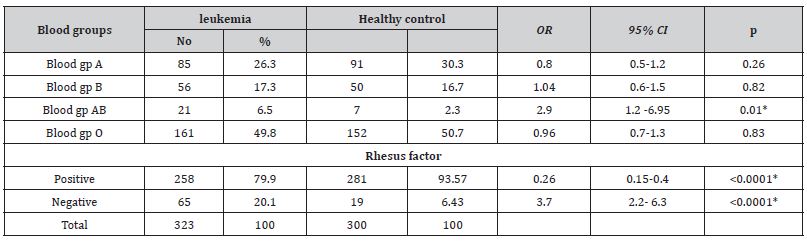
The mean ± SD age of all leukemia cases was 7.96 ± 3.93 years. Most of the cases were in the age group 6-10 years (67.8%), followed by the age group 11-15 years (25.1%), while only 7.1% of the cases were in the age group 1-5 years. As for gender, slightly more of the cases were males (53.3%), while the percentage of females was 46.7% (male to female ratio = 1.14-1). Most of the patients were from rural areas counting 68.7% while only 31.3% were from urban areas (Table 1). Table 2, 3 shows the prevalence of leukemia type among children with childhood leukemia in Sana’a, Yemen, and most cases were ALL (83.3%) while AML was only 16.7%. There was a significant association between gp AB and the incidence of leukemia in children where the odds ratio was 2.3, 95% CI =1.2-6.95 and p value 0.01, while there was no significant association with other blood groups. Rh factor-positive was the protective factor against childhood leukemia versus rhesus-negative factor in which it was a highly significant factor for childhood leukemia with an associated odds ratio of 6.43, with a CI of 2.2–6.3, with a p-value of <0.0001 (Table 3).
Table 1: Age and gender distribution of children with childhood leukemia in Sana’a, Yemen.
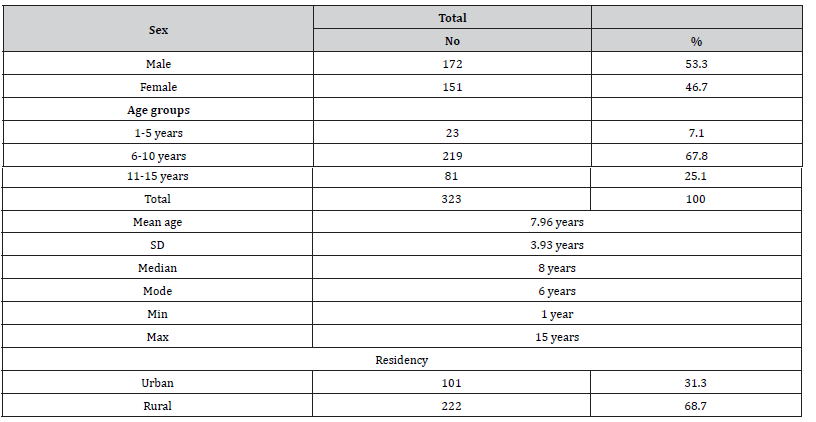
Table 2: Age and gender wise distribution of various types of leukemia’s among children suffering from childhood leukemia in Sana’a, Yemen.
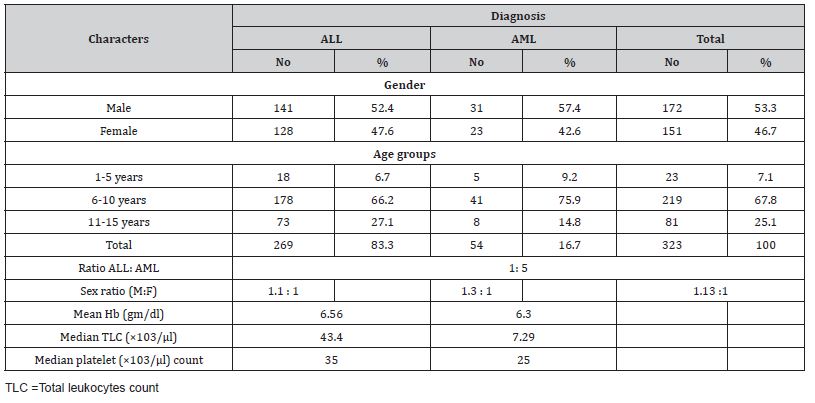
Table 3: The association of blood grouping and rhesus factors with childhood leukemia in Sana’a, Yemen.
Discussion
The mean ± SD age of all cases was 7.96 ± 3.93 years. Most of the cases were in the age group 6-10 years (67.8%), followed by the age group 11-15 years (25.1%), while only 7.1% of the cases were in the age group 1-5 years. As for gender, slightly more of the cases were males (53.3%), while the percentage of females was 46.7% (male to female ratio = 1.14-1). Information of the prevalence of leukemia in a population may envisage pathogenic hypotheses for disease control and aid in the effective management of leukemia and other malignant hematomas. In developing countries, particularly in Yemen, there is little information about the burden and patterns of haematological malignancies, particularly leukemia. In the current study, with regard to sex, the proportion of males was slightly more (53.3%), while the proportion of females was 46.7% (male-to-female ratio = 1.14-1). This finding is comparable to that from Africa, where the ratio of males to females is roughly equal, though females slightly predominate (1: 1.06) [16], but it differs from that of the United States, where the American Cancer Society predicts that there are about 5,690 new cases of leukemia in 2021, with 3,000 male cases and 2,690 female cases, as well as from that from Yemen, where the majority of cases were male (66.7%) and female cases were 33.3% only (male to female ratio = 2:1) [17]. The current findings of differing leukemia prevalence rates by gender are at odds with the notion that these differences must exist because of biological causes [18-21].
Leukemia may appear at all ages, from newborns to the elderly, but the distinctive forms have different age distributions [22]. In the current study, the mean age of ± SD for all cases was 7.96 ± 3.93 years and most of the cases were in the age group 6-10 years (67.8%) (Table 1). This is roughly similar to what has been reported elsewhere for pediatric leukemia where the mean age of pediatric leukemia cases was 6.0 years with a peak incidence at 6-10 years [23-25]. This differs from the leukemia hypothesis with age in which older children may develop leukemia more frequently than younger children due to advancing age, as many environmental exposures to carcinogens, irradiation, and malignant mutations due to clonal expansion occur more often [26, 27]. However, most of the younger children in the current study could be explained by the fact that prenatal and early life exposure is thought to be important determinants of childhood leukemia. Several mechanisms have been identified through which external and internal factors can influence the risk of developing leukemia in children. Exposure to a carcinogen or toxic substance early in a female’s life may cause permanent damage. Since no new oocytes are formed after birth and their maturation begins during pregnancy, the exposure that occurs during this critical time can be of great importance. During pregnancy, exposure to agents such as ionizing radiation may act directly while others may act indirectly by transporting the placenta. On the other hand, offspring may be exposed after birth to environmental exposure, either directly or indirectly [28].
The majority of the kids (68.7%) in Table 1 came from rural areas, so it’s possible that while they were living with their farmers’ parents, they were exposed to a variety of environmental factors. Even though they are not well understood, environmental factors affect the likelihood of acquiring leukemia. In Yemen, farming and plantations are the main forms of agriculture. In Gat, in particular, fruits and vegetable plantations are the main activity in the study area. This may result in the repeated use of chemicals like pesticides, herbicides, and fertilizers for agricultural activities, which will cause genetic mutations that confer leukemia [29].
Leukemia types were determined using the FAB classification method [18], Wright-stained morphological examination, and cytochemical staining with Sudan black B staining to differentiate the cell lineage. In this study, acute lymphocytic leukemia was the most common, accounting for 83.3% of the total, while acute myelogenous leukemia counted 16.7% (Table 2). This result was consistent with results from Ethiopia, Nepal, and Pakistan [16, 30], while it was contradictory with a study from Albania [31].
In the current study there was a significant association between blood group AB and the incidence of leukemia in children where the odds ratio was 2.3, 95% CI =1.2-6.95 and p value = 0.01 (Table 3). Our results are different from those reported by Kumar, et al. [32] Where no association was found between any type of blood group and any type of leukemia [32]. Also, our finding was different from that of Hansen, et al. [33] Where they found that there is an association between blood group B+ with leukemia having the largest number of leukemia patients in the blood group B+ [33, 34] Our significant association between blood group AB and leukemia is similar to that reported by Macmahon, et al. [35] Where the tendency of leukemia was shown to occur less in people with blood group O than with blood group AB [35]. Steinberg et al. [36] found no difference in ABO blood group distribution among acute leukemia patients compared to the general population [36]. Shirley and Desai reviewed several previously published data and found that there was no statistically significant difference in ABO blood group distribution in patients with acute leukemia when compared to respective controls in each study reviewed [37].
Rh-positive was the protective factor against childhood leukemia versus Rh-negative and was a highly significant factor in childhood leukemia with an associated odds ratio of 6.43, with a CI of 2.2–6.3, with a p-value <0.0001. Our finding is similar to the clinical investigation by Shahriari, et al. [38]. Where they found an association between the Rh-negative and leukemia. Daneshfard, et al. [39] came upon extensive incidental data in which, with the exception of one patient with ALL, all forty patients were Rh negative. Furthermore, it could be possible that rhesus antigen D negative has some potency in conducing ALL, accounting as a risk factor. On the other hand, if this antigen plays a role in the progression of the disease, the immune system might suppress the presentation of this antigen on the red blood cells through epigenetic or other mechanisms. In a report by Radhakrishnan, et al. [40], the authors presented two leukemia patients who had a blood type change during their leukemia treatment. The mechanism by which blood type changes in leukemic patients has not been clearly described, but it has been hypothesized that through epigenetic modifications to transcription- regulatory regions of the ABO gene in RBCs, malignant cells inhibit transcription antigens, thus changing blood group.
Limitation of the study
There were a number of limitations in this study. It was a retrospective, hospital based study. There was selection bias as all cases were the ones which presented to hospital. Hospital based study also does not account for the number of similar cases within the community, therefore, the estimates of relative prevalence of certain diseases cannot be generalized. The data was taken from his tory sheets of patients which were filled in by different residents, hence, they were not uniform.
Conclusion
ALL is the most common type of leukemia in Sana’a City. The ratio of males to females is almost equal, and young children are the most affected by leukemia. In the current study there was an association between leukemia and age group 6-10 years. This study showed a significant association between leukemia and ABO blood group and showed that AB blood group was associated with a higher risk of ALL (p-value < 0.01). The current study revealed that there are statistically significant between Rh-negative patients and developing leukemia in children. These findings also suggest the potential for using blood types as an epidemiological marker to identify population subgroups that are highly susceptible to certain cancers. With increasingly advanced diagnostic techniques, it is necessary to conduct more thorough research of pertinent components and predictors and to look into correlation factors with treatment regimens.
Acknowledgment
Thanks to the Pediatric Leukemia Unit at Kuwait Hospital, specifically to Dr. Abdulrahman Al-Hadi, a human doctor who spends his life, knowledge and time for children who suffer from this malignant disease.
Conflict of Interest
“No conflict of interest associated with this work”.
Author’s Contribution
The first author presented the data and the first, second and the third authors analyzed the data and wrote, revised and edited the paper.
Ethical Approval
Ethical approval was obtained from the Medical Research & Ethics Committee of the Faculty of Medicine and Health Sciences, Sana’a University. All data, including patient identification were kept confidential.
References
- Abdulganiyu (2016) Distribution of ABO and Rh (D) blood groups and associated traits: a study of the College of Nursing and Midwifery, Msc Thesis Dissertation, Kogi State, Obangede.
- Mohandas N Narla A. (2005) Blood group antigens in health and disease. Current Opinion in Hematology 12(2): 135-
- Denomme GA (2011) Molecular basis of blood group expression. Transfusion and Apheresis Science 44 (1): 53-
- Chandra T, Gupta A (2012) Association and distribution of hypertension, obesity and ABO blood groups in blood donors Iranian. Iran J Ped Hematol Oncol 2(4): 1-6.
- Abegaz SB (2021) Human ABO Blood Groups and Their Associations with Different Diseases. Biomed Res Int 2021: 6629060.
- Sandler SG, Mallory D (1995) Biological functions of blood groups in health and disease. Haematologia (Budap) 27(1): 1-
- Green C (1989) The ABO, Lewis and related blood group antigens; a review of structure and biosynthesis. FEMS Microbiol Immunol 1(6-7): 321-
- Ewald R, Sumner S (2016) Blood type biochemistry and human disease. Wiley Interdiscip Rev Syst Biol Med 8(6): 517–535.
- Daniels G (2002) Human Blood Groups. in Blackwell Science, Oxford, (2nd).
- Yamamoto F, Cid E, Yamamoto M, Blancher A (2012) ABO Research in the Modern Era of Genomics. Transfus Med Rev 26(2): 103-
- Cartron JP, Colin Y (2001) Structural and functional diversity of blood group antigens. Transfus Clin Biol 8(3): 163-
- Bassan R, Hoelzer D (2011) Modern therapy of acute lymphoblastic leukemia. J Clin Oncol 29(5): 532-543.
- Cohen W, Castelli C, Alessi MC, Aillaud MF, Bouvet S, et al. (2012) ABO blood group and von Willebrand factor levels partially explained the incomplete penetrance of congenital thrombophilia. Arterioscler Thromb Vasc Biol 32(8): 2021-2038.
- Reid ME, Lomas-Francis C, Olsson ML (2012) The blood group antigen facts book: Academic Press.
- Wu O, Bayoumi N, Vickers M, Clark P (2008) ABO (H) blood groups and vascular disease: a systematic review and meta‐ J Thromb Haemost 6(1): 62-69.
- Woldeteklehaymanot Kassahun, Girum Tesfaye, Lealem Gedefaw Bimerew (2020) Prevalence of Leukemia and Associated Factors among Patients with Abnormal Hematological Parameters in Jimma Medical Center, Southwest Ethiopia: A Cross-Sectional Study. Hindawi. Advances in Hematology 2014152: 1-7.
- Monya Abdullah Yahya El-Zine, Abdulrahman M Alhadi, Abdulrahman A IshaK, Hassan A Al-Shamahy Prevalence of Different Types of Leukemia and Associated Factors among Children in Children's Cancer Units at Al-Kuwait Hospital, Sana'a City: A Cross-Sectional Study, New Medical Innovations and Research 2(4).
- Eys JV, Pullen J, Head D (1986) The French-American-British (FAB) classification of leukemia. The pediatric oncology group experience with lymphocytic leukemia. Cancer 1;57(5): 1046-1051.
- Solomon Tessema M, Mahlet Kifle H, Asefa M (2018) Estimates of Cancer Incidence in Ethiopia in 2015 Using Population-Based Registry Data. J Glob Oncol.
- (2010) Ireland National Cancer Registry Cancer Trends; Leukaemia, National Cancer Registry Ireland, Dublin, Ireland.
- Hodgson CS (2016) Blood Cancer in Canada Facts and Statistics, Leukemia and Lymphoma Society of Canada, Toronto, Canada.
- Juliusson G, Hough R (2016) Leukemia Prog Tumor Res 43: 87-100.
- American Cancer Society (2018). Acute Myeloid Leukemia (AML) Subtypes and Prognostic Factors, American Cancer Society Inc. Atlanta, GA, USA.
- Australia Cancer Society Leukaemia Statistics, Australia Cancer Society, 2017.
- American Cancer Society (2017) Cancer Facts and Figures, American Cancer Society Inc. Atlanta, GA, USA.
- Konieczny J, Arranz L (2018) Updates on old and weary haematopoiesis Int J Mol Sci 19(9): 2567
- Cagnetta A, Soncini D, Orecchioni S (2018) Depletion of SIRT6 enzymatic activity increases acute myeloid leukemia cells' vulnerability to DNA-damaging agents. Haematologica 103(1): 80-90.
- Lightfoot T (2005) Aetiology of childhood leukemia, Bioelectromagnetics 26(S7): S5-S11.
- Sinner PJ, Cerhan JR, Folsom AR & Ross JA (2005) Positive association of farm or rural residence with acute myeloid leukemia incidence in a cohort of older women. Cancer Epidemiol Biomarkers Prev 14(10): 2446-2448.
- Khan, A., Ahmad, M., & Khan, S (2018) Analysis of clinic-epidemiological features of leukemia at a tertiary care facility in Peshawar. Journal of Medical Sciences 26(1): 41-45.
- Pepa E (2017) Prevalence of leukemia in Albania in the recent year. Inter-displinary Journal of Research and Development 4(2): 1-5.
- Kumar S, Mahto N, Bharti A, Meena LP (2020) Hematological malignancies in relation with ABO blood group at a teaching hospital, Varanasi, India. J Family Med Prim Care 9(5): 2309-2312.
- Hansen NE, Karle H, Jensen OM (1983) Trends in the incidence of leukemia in Denmark, 1943‑1977: An epidemiologic study of 14,000 patients. J Natl Cancer Inst 71(4): 697‑
- Modak H, Kulkarni SS, Kadakol GS, Hiremath SV, Patil BR, et al. (2011) Prevalence and risk of leukemia in thematic‑ethnic population of North Karnataka. Asian Pac J Cancer Prev 12(3): 671‑
- Macmahon B, Folusiak JC (1958) Leukemia and ABO blood group. Am J Hum Genet 10(3): 287‑2
- Steinberg AG, Steinfeld JL (1960) The genetics of acute leukemia in children. Cancer 13(5): 985-99.
- Shirley R, Desai R (1965) Association of leukaemia and blood groups. J Med Genet 2(3): 189-191.
- Shahriari M, Dehghankhalili S, Heiran A, Daneshfard B (2021) Negative Rhesus Antigen D in Childhood Leukemia: A Risk Factor or a Defense Mechanism. Iran J Public Health 50(5): 1077-1078.
- Daneshfard B, Shahriari M, Heiran A (2020) Effect of chamomile on chemotherapy-induced neutropenia in pediatric leukemia patients: A randomized triple-blind placebo-controlled clinical trial. Avicenna J Phytomed 10(1): 58-69.
- Radhakrishnan V, Mishra S, Bhaskar N, Sagar T (2016) Blood Group Change in Pediatric Leukemia: A Rare Phenomena. Indian J Pediatr 83(8): 874.
-
El-Zine MAY, Amer Ali MA and Al-Shamahy HA*. The Association of ABO Blood Types with Childhood Leukemia. Arch Clin Case Stud. 3(4): 2023. ACCS.MS.ID.000566.
-
ABO blood, Leukemia, Immune systems, Physiology, Platelets, Genetic changes, Blood types, Clinical symptoms, Pregnancy.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






