 Case Report
Case Report
Safety and Efficacy of Postoperative Cefovecin Prophylaxis in Dogs Undergoing Clean Orthopedic Procedures
Cássio Ricardo Auada Ferrigno1*, Bianca Fiuza Monteiro2, Aline Schafrum Macedo3, Mario Ferraro4,Vanessa Couto de Magalhaes Ferraz5 and Márcio Poletto Ferreira6
1College of Veterinary Medicine, The University of Tennessee, College of Veterinary Medicine, Knoxville, Tennessee, USA
2Surgery, Animal Care Ipiranga Animal Hospital, Sao Paulo, Sao Paulo, Brazil
3Department of Veterinary Surgery, School of Agricultural and Veterinary Sciences, Jaboticabal, Sao Paulo, Brazil
4Mobile Veterinarian, Sao Paulo, Brazil
5Knox Veterinary Surgery
6Medicina Animal, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil
Cássio Ricardo Auada Ferrigno, College of Veterinary Medicine, The University of Tennessee, College of Veterinary Medicine, Knoxville, Tennessee, USA
Received Date: June 14, 2024; Published Date: June 24, 2024
Abstract
Background: Prophylactic antibiotic therapy is a modern practice that aims to reduce the incidence of Surgical Site Infections (SSI) with special importance on surgeries with implanted medical devices, like orthopedic procedures. Cefovecin (ConveniaTM) is a unique long-acting thirdgeneration cephalosporin that can maintain peak plasma levels for up to 14 days with a single parenteral injection. Cefovecin is known to be safe and effective as a treatment for infections of the skin, urinary, genital and respiratory tracts of companion animals. It can treat canine periodontal diseases as well. This study aimed to validate the use of cefovecin as postoperative prophylactic antibiotic treatment in dogs that underwent clean orthopedic surgeries compared to oral cephalexin.
Methods: Medical records of 105 dogs that underwent clean orthopedic surgical procedures, regardless of implant application, were retrieved from a Veterinary Teaching Hospital and enrolled in the retrospective study. They were divided into groups according to the postoperative antibiotic prophylaxis: Fifty-one received a single subcutaneous cefovecin injection, and 54 received a twice-daily regimen of oral cephalexin.
Results: Mean body weight of cephalexin-treated animals was 29.25 ± 13 kg, statistically heavier (p = 0.00003138) than the cefovecin-treated dogs (13.36 ± 13.22 kg). Adverse effects occurred more frequently on cephalexin-treated animals (n = 18; p = 0.004394), mainly vomiting (n=17). Overall SSI incidence in Cefovecin and Cephalexin groups were 5.88% and 11.11%, respectively.
Conclusions: Cefovecin, administrated in a single sc dose (8 mg/kg of bw) was an effective postoperative antibiotic prophylactic treatment on 94.11% (48/51) of the clean canine orthopedic surgeries included in this study. Compared to oral cephalexin treatment, cefovecin caused fewer adverse reactions.
Keywords: Surgical site infection; orthopedic surgery; antimicrobial; cefovecin
Introduction
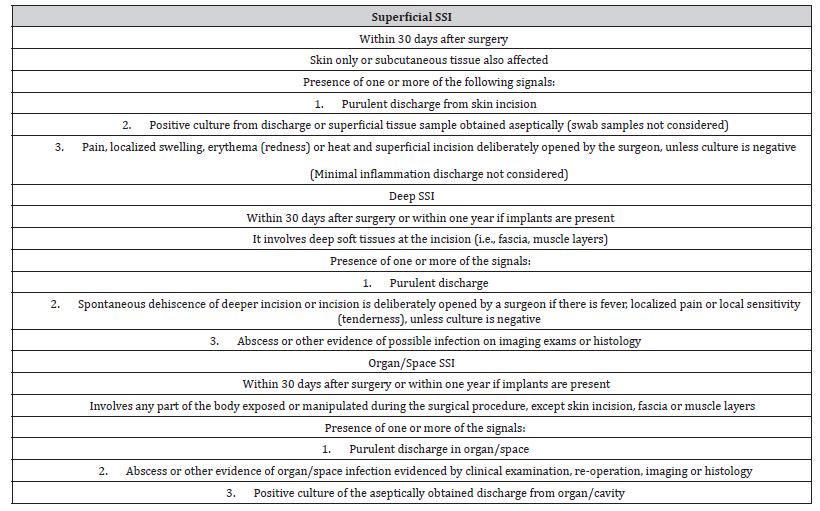
Surgical Site Infections (SSI) are a major concern in modern medicine and an inherent risk of any surgery. On orthopedic procedures, SSI can lead to severe complications such as osteomyelitis. Antimicrobials to prevent SSI are called prophylactic antibiotic therapy or antibiotic prophylaxis. It is a common practice that minimizes surgical morbidity but does not prevent all types of infections [1]. Clear guidelines for defining SSI have been developed for human patients [2-4] and adapted to veterinary medicine [5-7]. According to these guidelines, SSI are divided into three categories: superficial, deep, and organ/space SSI. Even when following strict protocols to maintain a sterile surgical environment and prevent infection, 81% of clean orthopedic surgeries still experience some level of contamination during the operation [8]. Orthopedic interventions carry a higher risk of infection due to the surgical complexity involved, which can cause significant damage to the soft tissue, as well as the length of the surgery and the use of metallic medical devices [9].
Bacteria in the surgical site can adhere to any implant’s surface and create surface-associated multicellular communities, known as biofilm. The composition and function of a biofilm varies depending on the specific microorganism involved, but it relies on a matrix created by the cells within it for its stability. This matrix, made up of extracellular polysaccharides, helps protect the biofilm from damage and allows the bacteria to remain dormant for extended periods of time, potentially leading to implant failure [10]. One of the major challenges in orthopedic surgery is the connection between implant placement and surgical site infections (SSI). This is because infections can negatively impact the healing and integration of the implant, potentially causing it to fail prematurely [11]. Postoperative antibiotic therapy can reduce 84% the risk of infection, thus protecting surgical sites of clean orthopedic procedures involving an implanted medical device [12,13]. Antibiotic prophylaxis is indicated for such surgeries and for procedures that last longer than two hours [14].
Cephalosporins are commonly used in human medicine, but they can cause hypersensitivity and anaphylactic reactions in people. Fortunately, these types of reactions seem to be less common in companion animals [15-18]. Possible adverse gastrointestinal effects, such as anorexia, diarrhea, and vomiting, may occur when taking this medication due to local gastric reaction and potential harm to intestinal flora. These effects can happen at any dosage but are more common at higher doses. Cephalexin is a commonly used first-generation drug in veterinary medicine. When taken orally, it is easily absorbed and has a 70 to 90% bioavailability. It is also distributed effectively in interstitial extracellular fluids [19]. This medication is effective in treating a range of bacterial infections that can impact the bones, respiratory system, skin, soft tissue, and urinary tract. The recommended therapeutic approach involves administering 10 to 30 mg/kg of body weight doses every six to twelve hours [20]. Cefovecin is a third-generation semi-synthetic cephalosporin with unique pharmacokinetic profile [21].
cephalosporin with unique pharmacokinetic profile [21]. It is formulated as a sterile aqueous solution for subcutaneous (sc) administration, with exceptionally long elimination half-life (approximately 5.5 days), low plasma clearance (0.76 ml/h/kg) and consistent maintenance of plasma drug levels for at least 14 days following a single sc dose [22]. The drug exhibited excellent in vitro activity across an extended spectrum of bacteria and was effective against Gram-positive pathogens, including Staphylococcus spp., Streptococcus spp. and Gram-negative pathogens as Escherichia coli. Similar to other β-lactam antibiotics, it exerts its bactericidal effect by inhibiting the synthesis of the peptidoglycan layer of the bacterial cell wall. Cefovecin is considered clinically safe for not causing severe adverse effects, such as anaphylactic or hypersensitivity reactions [23,24].
It does not display apparent drug interaction with different medications, including anesthetic’s, analgesics, antihistamines, ectoparaciticides, NSAIDs and tranquilizers. Cefovecin has already been proven effective for the treatment of superficial and deep pyoderma [25], urinary tract infections and for severe canine periodontal disease [26]. To the authors’ knowledge, this is the first study on the use of cefovecin as prophylactic SSI treatment in dogs that underwent orthopedic procedures. The aim of this study was to compare the effectiveness of cefovecin and oral cephalexin as postoperative prophylactic antibiotics for dogs undergoing clean orthopedic surgeries, with cephalexin being the standard antibiotic used in postoperative care for dogs. We identified the medical records of 105 dogs owned by clients who received orthopedic procedures at a Veterinary Teaching Hospital over 20 months. We divided them into groups according to whether they received antibiotics post-surgery. We collected data to determine the occurrence of surgical site infections and/or bone infections, as well as any potential side effects. Furthermore, we interviewed owners of patients who received cefovecin over the phone using a standard client satisfaction questionnaire.
Materials & Methods
Study Design and Inclusion Criteria
This was a retrospective observational study. Medical records were searched from client-owned dogs that underwent orthopedic surgeries in the Veterinary Teaching Hospital over a 20‐month period (between August 2013 and March 2015) who received either cefovecin or cephalexin as antimicrobial prophylaxis. All dog owners involved in the study were contacted by phone and asked to sign a consent form allowing their dogs’ data to be included in the study. Only three owners declined to give their approval, and their dogs’ data was subsequently withdrawn from the study. All surgical reports were retrieved, and only the patients that underwent clean orthopedic procedures (regardless of implanted medical device) were identified and assigned to groups based on antibiotic prophylaxis (only cefovecin and cephalexin were included).
All animals also received perioperative antibiotic prophylaxis of cephalothin (30 mg/kg bw; IV) 30 to 60 minutes prior to skin incision, with additional doses every 90 minutes until skin closure if necessary. Patients with incomplete medical records were considered not suitable for enrolment. Other exclusion criteria included: comorbidities or infections (i.e., dermatitis, otitis), contaminated wounds or open fractures, and history of treatment with local or systemic antimicrobial agents (within seven days prior to surgery or after surgery). If there was a record of any surgical site or personnel contamination during the procedure, the patient was also not enrolled. Data obtained from medical records included: patient ID number, age, gender, breed, body weight, affected limb(s), comorbidities, type of surgical procedure, antibiotic prophylaxis information (name, type, dose, route, and frequency of administration), concomitant medications, possible side effects and signs of SSI. Any other relevant information assessed on the postoperative follow-up was also included. Data retrieved was recorded into commercially available database software for further analysis.
Surgical Procedure Information
Experienced surgeons at the Teaching Facility performed all surgical procedures in a dedicated operating room for clean orthopedic procedures. The room adhered to strict sterilization standards and only authorized personnel with proper scrubs were allowed in. Some patients were given wound dressings without any topical medications, while others received a Robert Jones Bandage for 24 to 48 hours if necessary. The study examined patients who were discharged either on the same day or within one day of hospitalization following their surgical procedure. Specific recommendations and written instructions were provided to all the owners at the time of hospital discharge advocating cage rest, proper wound care and use of e-collar until suture removal. Postoperative clinical and radiographic re-assessments were scheduled according to each patient’s needs.
Surgical Site Infection Assessment Criteria
The classification of surgical sites followed the standard SSI definitions (Table 1). The categories included: normal, inflamed (but not infected), superficial SSI, deep SSI, and organ/space SSI. In cases where there were indications of bone infection, it was classified as organ/space SSI. Confirmation of osteomyelitis relied on radiographic findings such as soft tissue swelling, bone lysis, and/or sequestration, excessive periosteal reaction, and delayed or non-union. Positive culture of bone tissue was also considered.
Table 1: Surgical Site Infection (SSI) Definitions [2].
Assessment of owner satisfaction with cefovecin therapy
Owners of patients from cefovecin group were contacted by telephone and interviewed using a standard client satisfaction assessment questionnaire (Table 2), developed by the authors.
Statistical analysis
Standard statistical methods were used to describe and analyze quantitative data. A Shapiro-Wilk test was used to evaluate if the data was normally distributed. Possible relationships between age and gender were tested by chi-square (X2) analysis. Age and body weight were not normally distributed and were compared by a Wilcoxon test. Fisher’s exact test was used to evaluate the qualitative data as to type of procedure, adverse effects and possible intercurrences. Significance level was established at 5% (P <0.05). All statistical tests were carried out by use of a statistical software program (RStudio, Version 0.99.903 – © 2009-2016 RStudio, Inc.).
Results
Medical records of 105 dogs met the criteria and were enrolled in the study. They were placed in two groups according to the postoperative antibiotic prophylaxis they were given: Cefovecin (n=51) and Cephalexin (n=54). (Table 3) illustrates data from all patients from the study. The ages of the dogs in the cefovecintreated group ranged from four months to 14 years (mean ± sd = 4.97 ± 3.59y) and did not differ significantly (p = 0.2144) from cephalexin-treated group where they ranged from three months to 15 years (mean ± sd = 3.98 ± 3.01y). Mean body weight (bw) of animals placed on Cephalexin Group was 29.25 ± 13 kg, statistically heavier (p = 0.00003138) than those from Cefovecin Group (13.36 ± 13.22 kg). There were 50 females (24 on group Cefovecin and 26 on group Cephalexin) and 55 males (27 and 28 on groups Cefovecin and Cephalexin, respectively) on this study.
Table 2: Client Satisfaction Assessment Questionnaire for the owners of the Cefovecin group.

Table 3: SD: standard deviation; y: years; m: months; kg: kilogram.
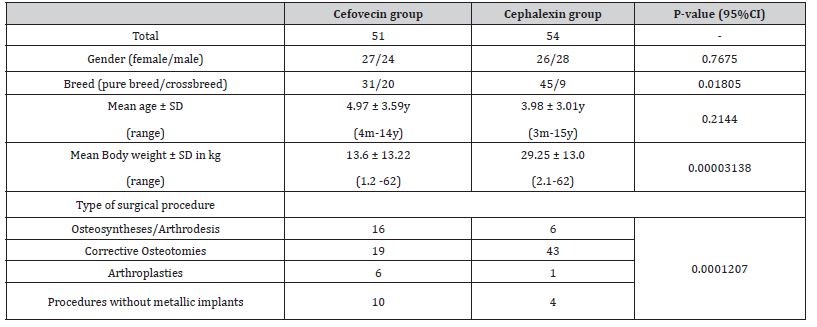
Cefovecin-treated patients received a single sc injection (8 mg/kg of bw; ConveniaTM, Pfizer Animal Health) at the end of the surgical procedure. Cephalexin-treated patients received a twicedaily dosing of 28.8 ± 3.58 mg/kg of bw (range: 21 to 36 mg/ kg) for about 9.5 ± 1.07 days after surgery (ranging from 7 to 10 days). The incidence of pure breed dogs in Cephalexin group was higher than on Cefovecin group (n = 45/51, p = 0.01805). Among them, Pit Bulls were the most prevalent (n=9; 16.6%), followed by Labrador Retrievers (n=8; 14.8%). Meanwhile Pinschers were the most frequent dogs in the Cefovecin group (n=10; 19.6%). The most prevalent surgical procedures of Cephalexin group were corrective osteotomies (n=43), which differed significantly from the procedures of Cefovecin group (p = 0.0001207). The procedures in the Cephalexin group consisted mainly of Tibial Plateau Leveling Osteotomies (TPLO) (79.62%) and one correction of bone deformity (1.85%). On the other hand, Cefovecin group had 17 TPLOs (33.13%) and two corrections of bone deformity (3.92%).
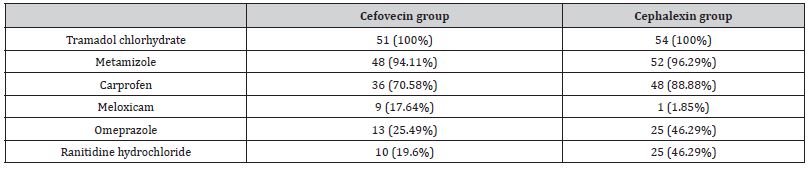
Two patients from the Cefovecin group had fractures in more than one limb but both osteosynthesis procedures counted as a single surgery (bilateral radius/ulna in one case and radius/ulna and femur in the other case). All procedures named as “arthroplasty” in Table 3 refer to correction of patellar luxation with tibial crest transposition and tension wire band, associated or not with additional techniques. Procedures without metallic implants were: extracapsular suture for cruciate disease (n=4; 7.84%); implant removal after bone healing (n=3; 5.88%), iliofemoral suture (n=1; 1.96%), acetabular denervation (n=1; 1.96%) and thoracolumbar hemilaminectomy (n=1; 1.96%) on Cefovecin group and acetabular denervation (n=3; 5.55%) and femoral head osteotomy (n=1; 1.85%) on Cephalexin group. Postoperative medications are listed on (Table 4). Isolated cases received specific medications such as oral lactulose, ondansetron, sucralfate, phenobarbital, spironolactone and gabapentin, chondroprotective nutraceuticals and metoclopramide.
Table 4: Concomitant medications.
Time for suture removal ranged from ten to 14 days. Side effects were more frequently encountered on cephalexin-treated animals (n=18/54, p = 0.004394), mainly intermittent vomiting (n=17). All adverse effects are described on (Table 5). Side effects led to a change in therapy in ten dogs from group Cephalexin. Six patients were discontinued of oral cephalexin therapy because of gastrointestinal side effects and were shifted to cefovecin treatment (one of them developed serious gastritis requiring supportive therapy with ranitidine hydrochloride, metoclopramide and sucralfate); two were shifted to a different antibiotic treatment. The last two patients simply had omeprazole added to the protocol, but overall treatment remained unaltered. On dog was not dosed according to instructions by owners due to compliance-issues case and also received a Cefovecin injection. A total of 15 surgical site intercurrences were documented on both groups, among them nine SSI cases, without statistical differences on either frequency (P = 0.7836) nor category (P = 0.346) between them, as described in Table 6.
Table 5: Number and Rate of side effects for both treatment groups.
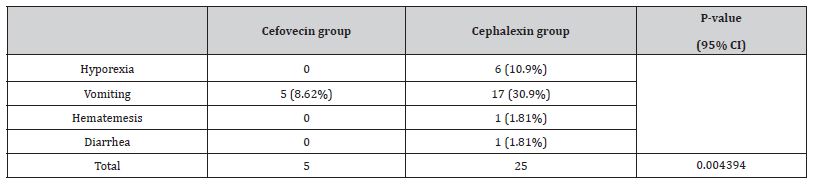
Table 6: Number and Rate of surgical site intercurrences/infection.
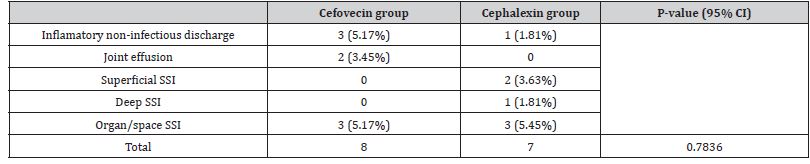
Four animals had incision abnormalities classified as noninfected inflammation and two cefovecin-patients (3.92%) developed non-infected joint effusion (after extracapsular sutures). One of them was later diagnosed with lymphoplasmacytic synovitis. Superficial SSI occurred on two Cephalexin-treated patients (3.63%) and a single deep SSI case was reported at the same group (1.81%). Organ/space SSI occurred in three patients from each group. All SSI cases from group cephalexin occurred after TPLO procedures. Superficial SSI was documented in two of these dogs (3.70%) and resolved spontaneously within two weeks. Deep SSI with purulent wound discharge and spontaneous suture dehiscence occurred on another patient (cephalexin treatment was prolonged for another 17 days, until second intention healing). There were no additional cases of SSI or osteomyelitis found in these dogs within one year after they received the implant device. Organ/space SSI occurred in three dogs from this group (5.55%), two of them with radiographic signs of osteomyelitis. Both animals achieved bone healing and clinical cure eventually, one of them required an antibiotic switch to amoxicillin with clavulanic acid.
The other patient had the implanted device removed after 9 months, but bacterial identification was not possible. The third patient tested positive to coagulase-positive Staphylococcus sp., sensitive to doxycycline. Complete follow-up was not possible due to lack of owner’s compliance on attending rechecks. Three organ/ space SSI cases (5.88%) were documented on cefovecin-treated animals. Two of them follow TPLOs and the other follows tibial osteosynthesis. The first TPLO SSI developed 60 days after surgery. Synovial fluid culture tested positive for strains of Staphylococcus sp. but histiocytic sarcoma was also diagnosed at histology exam. The patient was then referred to an oncologist. The second TPLO SSI had osteomyelitis confirmed at the 40th P.O. radiographic recheck but responded well to antibiotic therapy and 100 days after surgery remission of the radiographic signs were observed. The implant was removed eight months later. The third case occurred after tibial osteosynthesis was performed with a Dynamic Compression Plate, but proper post-operative was not provided due to owner’s compliance issues.
The case evolved poorly to implant failure within 30 days. During the revision procedure, a locking plate with an autogenous cortical graft was applied, but the implant failed again after 11 months. At the second revision surgery, a circular external skeletal fixator (ESF) apparatus was placed, and the culture tested positive for strains of Pseudomonas sp., sensitive to enrofloxacin. Complete follow-up was not achieved because the owner skipped rechecks. As to cefovecin-treated pet owners’ satisfaction assessment, telephonic contact reached 72.54% of the owners (31/51). The remaining could not be contacted despite several attempts. Among those who answered, 22.58% (n=7) could not recall the postoperative medications their pet received. The remaining 24 owners were then asked the following questionnaire items. The average cefovecin-treatment satisfaction score given was 9.7 (range 7 to 10). All the clients stated that they would choose the same medication again if given the chance. Fourteen clients (45.16%) reported regular difficulties in handling their pet’s oral medication doses.
Discussion
Cephalexin was chosen as positive control in this study because it is considered a suitable comparative cephalosporin. It is frequently prescribed and effective for canine orthopedic conditions. Dosing and frequency of administration were within the recommended literature guidelines. Oral dosing might be challenging for some pet owners, especially if they have non-cooperative animals or if they are faced with long-period treatments, such as antibiotics. That fact inexorably leads to compliance issues with correct dosing and may lead to multidrug-resistant bacteria. Cefovecin addresses the client’s difficulties in handling oral medications since a single parenteral dose might account for an entire treatment course, causes less medication-related stress on the treated animals and ensures the maintenance of plasma concentrations during the whole treatment interval, exerting its action at the infection site. Although not contemplated in this study, cats also benefit greatly from cefovecin treatment. Given the temperamental characteristics of this species, handling their oral doses might be even more challenging than dogs. Some irascible dogs’ owners might face the same problem, which indeed happened in our study and required antibiotic switch from oral cephalexin to injectable cefovecin.
In fact, according to the client’s opinion assessed by the questionnaire, difficulties in oral handling occurred 45.16% of the times. Body weight difference in between the two treatment groups was significant. Even the contrast between the most frequent breeds per group was hallmarked (Pinschers and Pit Bulls represented cefovecin and cephalexin groups respectively). The authors suggest that this may be a possible cost-issue that limits cefovecin use in larger dogs, as has been suggested previously [27,28]. Since cefovecin pricing is strictly based on the prescribed volume according to body weight, most large-breed-dog owners are reluctant in engaging this high-cost treatment. Cefovecin, an antibiotic, was found to be safe for use in this study as there were no reported drug interactions when taken with other medications. Adverse side effects were noted in less than 9% of cases and were primarily related to gastrointestinal issues such as vomiting. It cannot be confirmed that cefovecin was the sole cause of these symptoms as other drugs were also being taken during the same period.
On the other hand, animals treated with cephalexin had more notorious gastrointestinal adverse effects such as vomiting, anorexia and diarrhea, similar to previous studies [29]. In our study, cephalexin treatment had to be suspended or switched on 18.18% of the patients due to these intercurrences, affecting thus the drug’s prophylactic effect. Cefovecin can address such problems due to its parenteral administration, avoiding these types of undesirable reactions that can undermine the antibiotic’s therapeutic efficacy and predispose SSI and antibiotic resistance [30]. There were no serious adverse events, suspected adverse drug reactions nor local reactions attributed to treatment with Cefovecin in our retrospective analysis, just like evidence from other studies, highlighting the drug’s safety profile. Some injectable cephalosporins (as cephalothin) may, in fact, cause injection site reactions as moderate pain, heat and swelling, but they were not documented in our study. Inflammation is inevitable after surgery and postoperative inflammatory responses can vary greatly between animals. Infection was ruled out from the cases with incisional abnormalities in this study
If mild infection occurred at the inflammatory wound discharge, it was self‐limiting and did not evolve to SSI. The same is true to the cases that joint effusion’s culture tested negative, so they had uninfected, but inflamed, joints and incisions. SSI occurs in up to 18.2% of dogs that undergo clean orthopedic procedures [31-33], and patients that receive metallic implants are at 5.6 times greater risk. In our study the overall SSI incidence was 5.88% for cefovecintreated animals and 11.11% in cephalexin-treated animals. We believe that the higher SSI incidence on Cephalexin Group is associated to TPLO procedures, since those animals underwent mainly this type of procedure and it is known that SSI incidence increases with the appliance of a metallic implant. We believe that body weight affected this parameter since most of large breed dogs that come to our service have cruciate disease and undergo TPLO procedures. It’s important to note that our study has some limitations. It was conducted retrospectively, relying solely on medical records which may be incomplete or misinterpreted.
Additionally, there was no standardized surgery type or followup period. It’s recommended to have a one-year postoperative surveillance if a metallic implant is present [34,35]. It is known that prophylactic postoperative antibiotics are effective against SSI. Cefovecin not only has the same effect but has the advantage of avoiding owner’s compliance issues in handling oral doses that could undermine the treatment efficacy. Thus, we emphasize that further studies and controlled trials should be performed on the efficacy of cefovecin-treatment in orthopedic procedures. Owners interviewed in this study were highly satisfied with cefovecintreatment mainly due to the long-acting effect that frees the clients from handling oral doses and avoid managing medication-related stress caused in their pets. That should be the target reached by future studies for the development of new long-lasting antimicrobial formulations [36]. This study was not intended to analyze whether the antibiotic regimens were correctly applied in the cases enrolled or to promote the indiscriminate use of prophylactic antibiotics in all orthopedic procedures.
The authors reinforce the importance of following literature guidelines and personal clinical experience in the decision-making process as to utilize or not prophylactic antibiotics. That should be part of the surgical planning process allied to adherence to wellaccepted and rigid surgical field sterility standards. One should keep in mind that antibiotics must not compensate for poor sterility standards or bad technique. Minor orthopedic procedures that do not require implants (or utilize minor implants) should be carefully evaluated as to the necessity of prophylactic antibiotic therapy.
Conclusion
In this study, administering a single subcutaneous dose of Cefovecin (8 mg/kg of body weight) proved to be an effective postoperative antibiotic prophylactic treatment for 94.11% (48/51) of dogs who underwent clean orthopedic surgeries. Compared to oral cephalexin treatment, Cefovecin resulted in fewer adverse reactions and was deemed safe for clinical use in dogs.
Acknowledgement
None.
Conflict of Interest
No conflict of Interest.
References
- Steven C Budsberg, JA Kirsch (2001) Antibiotic prophylaxis in veterinary orthopaedic surgery. Vet Comp Orthop Traumatol 14(4): 185-189.
- TC Horan, RP Gaynes, WJ Martone, WR Jarvis, TG Emori (1992) CDC definitions of nosocomial surgical site infections, 1992; A modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13(10): 606-608.
- AJ Mangram, TC Horan, ML Pearson, LC Silver, WR Jarvis (1999) Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20(4): 250-278.
- Sítio cirurgico (2009) Critérios Nacionais de Infecções relacionadas à assistência à saúde [Internet]. Agência Nacional de Vigilância Sanitária pp. 19.
- Ryen Turk, Ameet Singh, J Scott Weese (2015) Prospective Surgical Site Infection Surveillance in Dogs. Vet Surg 44(1): 2-8.
- Budsberg SC (2012) Osteomyelitis. Tobias K, Johnston SA, Eds., Vet Surg small Anim. 1st edition, Philadelphia, Saunders, USA pp. 669-675.
- Bilgen Sen Z, Kilic Nuh (2012) Posttraumatic Bacterial Infections in Extremities before and after Osteosynthesis in Dogs. Acta Sci Vet 40(2): 1033.
- Natalia Andrade, Chad W Schmiedt, Karen Cornell, MaryAnn G Radlinsky, Lauren Heidingsfelder, et al. (2016) Survey of Intraoperative Bacterial Contamination in Dogs Undergoing Elective Orthopedic Surgery. Vet Surg 45(2): 214-222.
- Bubenik LJ, Smith MM (1993) Orthopaedic Infections. Slatter DH, Eds., Textb Small Anim Surg. Philadelphia, Saunders, USA. PP. 1862-1974.
- Jonathan P Bray, Andrew Kersley, Warwick Downing, Katherine R Crosse, Andrew J Worth, et al. (2017) Clinical outcomes of patient-specific porous titanium endoprostheses in dogs with tumors of the mandible, radius, or tibia: 12 cases (2013–2016). J Am Vet Med Assoc 251(5): 566-579.
- PA Suci, JD Vrany, MW Mittelman (1998) Investigation of interactions between antimicrobial agents and bacterial biofilms using attenuated total reflection Fourier transform infrared spectroscopy. Biomaterials 19(4-5): 327-339.
- Kumpanart Soontornvipart, A. NEâAS, Dvorak M (2003) Effects of Metallic Implant on the Risk of Bacterial Osteomyelitis in Small Animals. Acta Vet Brno 72(2): 235-247.
- Andrea Pratesi, Andrew P Moores, Ciara Downes, James Grierson, Thomas W Maddox (2015) Efficacy of Postoperative Antimicrobial Use for Clean Orthopedic Implant Surgery in Dogs: A Prospective Randomized Study in 100 Consecutive Cases. Vet Surg 44(5): 653-660.
- Grant GR, Olds RB (2007) Tratamento das fraturas expostas. Man Cir pequenos animais. 3rd ed. São Paulo: Manole PP.1793-1798.
- MR Stegemann, J Sherington, S Blanchflower (2006) Pharmacokinetics and pharmacodynamics of cefovecin in dogs. J vet Pharmacol Ther 29(6): 501-511.
- Kietzmann M, Nolte I, Strothmann-Luerssen A, Grunau B, Scharer V (1992) Tolerance and pharmacokinetics of cephalexin in cats after oral administration. J Small Anim Pract 33: 521-525.
- Mason IS, Kietzmann M (1999) Cephalosporins–pharmacological basis of clinical use in veterinary dermatology. Vet Dermatol 10(3): 187-192.
- (2003) USP Veterinary Pharmaceutical Information Monographs - Antibiotics. J Vet Pharmacol Ther 2: 1-271.
- Caprile KA (1988) The cephalosporin antimicrobial agents: a comprehensive review. J Vet Pharmacol Ther 11(1): 1-32.
- RJ Lunke, RH Fitzgerald, JA Washington (1981) Pharmacokinetics of Cefamandole in Osseous Tissue. Antimicrob Agents Chemother 19(5): 851-858.
- MR Stegemann, CA Passmore, J Sherington, CJ Lindeman, G Papp, et al. (2006) Antimicrobial Activity and Spectrum of Cefovecin, a New Extended- Spectrum Cephalosporin, against Pathogens Collected from Dogs and Cats in Europe and North America. Antimicrob Agents Chemother 50(7): 2286-2292.
- Passmore CA, J Sherington, MR Stegemann (2007) Efficacy and safety of cefovecin (Convenia) for the treatment of urinary tract infections in dogs. J Small Anim Pract 48(3): 139-144.
- MR Stegemann, Coati N, Passmore CA, J Sherington (2007) Clinical efficacy and safety of cefovecin in the treatment of canine pyoderma and wound infections. J Small Anim Pract 48(7): 378-386.
- Joseph D Palamara, Jennifer J Bonczynski, Jason M Berg, Philip J Bergman (2016) Perioperative Cefovecin to Reduce the Incidence of Urinary Tract Infection in Dogs Undergoing Hemilaminectomy. J Am Anim Hosp Assoc 52(5): 297-305.
- Robert Six, Judy Cherni, Robert Chesebrough, Dawn Cleaver, Cindy J Lindeman, et al. (2008) Efficacy and safety of cefovecin in treating bacterial folliculitis, abscesses, or infected wounds in dogs. J Am Vet Med Assoc 233(3): 433-439.
- Henry Giboin, Csilla Becskei, Jacky Civil, Michael Stegemann (2012) Safety and Efficacy of Cefovecin (Convenia<sup>®</sup>) as an Adjunctive Treatment of Periodontal Disease in Dogs. Open J Vet Med 2(3): 89-97.
- Vicki J Adams, John R Campbell, Cheryl L Waldner, Patricia M Dowling, Cindy L Shmon (2005) Evaluation of client compliance with short-term administration of antimicrobials to dogs. J Am Vet Med Assoc 226(4): 567-574.
- A Mateus, DC Brodbelt, N Barber, KDC Stärk (2011) Antimicrobial usage in dogs and cats in first opinion veterinary practices in the UK. J Small Anim Pract 52(10): 515-521.
- S Toma, S Colombo, L Cornegliani, P Persico, M Galzerano, et al. (2008) Efficacy and tolerability of once-daily cephalexin in canine superficial pyoderma: An open controlled study. J Small Anim Pract 49(8): 384-391.
- Joseph M Blondeau, Shantelle D Shebelski (2016) Comparative in vitro killing of canine strains of Staphylococcus pseudintermedius and Escherichia coli by cefovecin, cefazolin, doxycycline and pradofloxacin. Vet Dermatol 27(4): 267-e63.
- Yap FW, Calvo I, Smith KD, Parkin T (2015) Perioperative risk factors for surgical site infection in tibial tuberosity advancement: 224 stifles. Vet Comp Orthop Traumatol 28(3): 199-206.
- Savicky R, Beale B, Murtaugh R, Swiderski-Hazlett J, Unis M (2013) Outcome following removal of TPLO implants with surgical site infection. Vet Comp Orthop Traumatol 26(4): 260-265.
- Gallagher AD, Mertens WD (2012) Implant Removal Rate from Infection after Tibial Plateau Leveling Osteotomy in Dogs. Vet Surg 41(6): 705-711.
- Alan D Tice, Pamela A Hoaglund, David A Shoultz (2003) Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother 51(5): 1261-1268.
- Weese JS (2008) A review of post-operative infections in veterinary orthopaedic surgery. Vet Comp Orthop Traumatol 21(2): 99-105.
- Morena Bernadette Wernick, Cedric R Müntener (2010) Cefovecin: A New Long-acting Cephalosporin. J Exot Pet Med. Elsevier Inc 19(4): 317-322.
-
Cássio Ricardo Auada Ferrigno*, Bianca Fiuza Monteiro, Aline Schafrum Macedo, Mario Ferraro,Vanessa Couto de Magalhaes Ferraz and Márcio Poletto Ferreira. Safety and Efficacy of Postoperative Cefovecin Prophylaxis in Dogs Undergoing Clean Orthopedic Procedures. Arch Clin Case Stud. 4(1): 2024. ACCS.MS.ID.000579.
-
Surgical site infection; orthopedic surgery; antimicrobial; cefovecin; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






