 Mini Review
Mini Review
Gemcabene as a Potential Therapeutic for NASH: Lessons Learnt from its Clinical Trials in Dyslipidemia
Daniela Carmen Oniciu*1,2 and Jennifer Lynn Myers2
1Department of philosophy, university of Florida, Gainesville, FL, USA
2Department of Pharmacy, creative Pharma Advisors LLC, Gainesville, FL, USA
Daniela Carmen Oniciu, Department of philosophy, university of Florida, Gainesville, FL, USA.
Received Date: June 27, 2020; Published Date: July 10, 2020
Abstract
Elevated hepatic lipogenesis and inflammation are associated with the progression of non-alcoholic steatohepatitis (NASH) and cardiovascular disease. Gemcabene, a small molecule in development for dyslipidemia, reduces plasma very low-density lipoprotein cholesterol (VLDL-C), lowdensity lipoprotein cholesterol (LDL-C), triglycerides (TG) and C-reactive protein (CRP) in animal models and humans. An analysis of the clinical information available for gemcabene and translational research substantiates the potential of gemcabene as a therapeutic agent for NASH. Specifically, gemcabene showed positive effects in clinical trials in severe hypertriglyceridemia and in subsets of obese, diabetic patients, particularly reductions in triglycerides, cholesterol and proatherogenic and inflammation markers. In nonclinical models, gemcabene showed significant improvement in non-alcoholic fatty liver disease (NAFLD) activity and fibrosis scores and affected beneficially the hepatic expression of many lipid regulating and inflammatory genes.
Keywords:Gemcabene; Dyslipidemia; Hypercholesterolemia; Hypertriglyceridemia; Hypertriglyceridemia; Severe hypertriglyceridemia; SHTG; Cardiovascular risk; Non-Alcoholic Fatty Liver Disease; Non-Alcoholic Steatohepatitis; NAFLD; NASH
Abbreviations:TG: Triglycerides; LDL-C: Low –Density Lipoprotein; Non-HDL-C: Non-High-Density Cholesterol; VLDL-C: Very Low-Density Lipoprotein Cholesterol; Apo: apolipoprotein; HSCRP: High-sensitivity C Reactive Protein; SAA: Serum Amyloid A
Introduction
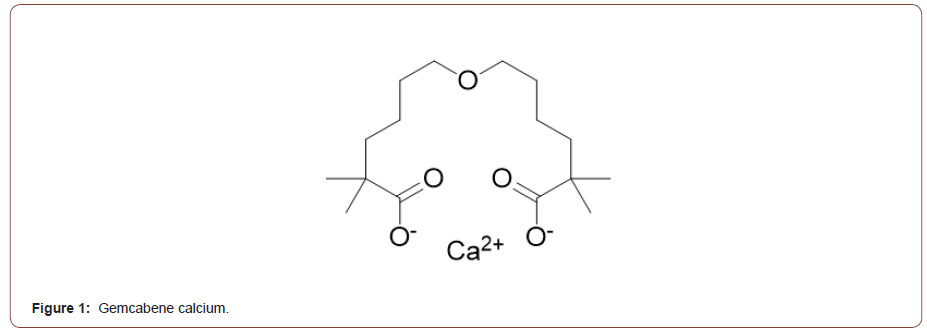
Several new or emerging drug candidates for Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis (NAFLD / NASH) owe their existence to repurposed dyslipidemia clinical programs built on the triglyceride (TG) and inflammation lowering properties and liver-directed mechanism of action of these agents. Revisited drug candidates for NAFLD/NASH were previously shown to reduce low-density lipoprotein cholesterol (LDL-C), triglycerides (TGs), and lipoprotein[a] (Lp[a]) in humans and reduce steatosis and fibrosis in nonclinical models [1]. By this approach, among new agents advancing towards late development in dyslipidemia, gemcabene may be of particular interest for NASH [2-4]. Gemcabene is a small molecule dialkyl ether having 2 terminal gem-dimethyl carboxylate moieties, formulated as its monocalcium salt (Figure 1).
The molecule was discovered at Parke Davis [5-7] and its clinical program as a lipid-lowering drug developed by Pfizer in the 2000s was based on its high-density lipoprotein cholesterol (HDL-C) elevating and triglyceride lowering properties [8,9]. Gemcabene has been evaluated at Pfizer as an oral therapy for the treatment of patients with hypercholesterolemia or hypertriglyceridemia who are unable to achieve normal levels of low-density lipoprotein cholesterol (LDL-C) or triglycerides (TGs) with currently approved therapies (e.g., statins and fibrates, and fish oils, respectively). In 2014, Gemphire Therapeutics acquired the rights to continue gemcabene development as a once-daily treatment for mixed dyslipidemia and for cardiometabolic rare diseases: homozygous familial hypercholesterolemia (HoFH) [10] and Familial Partial Lipodystrophy (FPLD) [11], a rare genetic disorder characterized by an abnormal distribution of adipose tissue including the development of a fatty liver. Recent nonclinical, clinical and translational research on the efficacy of gemcabene support the study of gemcabene in additional therapeutic indications: heterozygous FH (HeFH), hypertriglyceridemia (HTG), severe hypertriglyceridemia (SHTG), and Non-Alcoholic Fatty Liver Disease / Non-Alcoholic Steatohepatitis (NAFLD/NASH).
From Laboratory to Clinical Research
Early nonclinical studies with gemcabene evidenced that its mechanism of action is primarily directed to the reduction of de novo lipogenesis. In vitro experiments in primary rat hepatocytes showed that treatment with gemcabene significantly and dosedependently decreased de novo cholesterol (CHOL) and triglyceride (TG) synthesis at concentrations of 10 μM and 30 μM [5,6]. Also in vitro, gemcabene lowers pro-inflammatory acute-phase protein, C-reactive protein (CRP), by an NF-κB-mediated transcriptional mechanism (evidenced in primary human coronary artery endothelial cells) [12] and also suppresses IL-6- and IL-1β-induced CRP production (evidenced in hepatoma cells) [13]. Gemcabene‘s PPAR activity was shown to be irrelevant by an in vitro assay in a subset of human PPAR responsive genes compared to TG lowering agents gemfibrozil and fenofibrate [14], this observation may be correlated with differences reported in anti-inflammatory and cell signaling properties between gemfibrozil and gemcabene, with higher inflammation inhibition for the latter.
In vivo, in Sprague Dawley rats, a model of diabetes and obesity, gemcabene significantly reduced hepatic TGs (about 70%), similar to reductions seen with the current therapeutic agent gemfibrozil [6]. In the LDL receptor deficient mouse, a model of HoFH, gemcabene significantly reduced LDL-C alone (55%) and in combination with atorvastatin (72%), and inhibited progression of atherosclerosis, indicating a mechanism independent of the LDL receptor [15]. Overall, in animal models of dyslipidemia, gemcabene improves the clearance of very low-density lipoproteins (VLDLs) in the plasma, inhibits the de novo production of CHOL and TG in the liver, and downregulates hepatic acetyl-CoA carboxylase 1 (ACC1) and apolipoprotein C-III (apoCIII) [16-18].
Table 1: Summary of Clinical Findings with Gemcabene: Mean Percent Changes in Lipids, Lipoprotein Parameters and Inflammatory Markers.
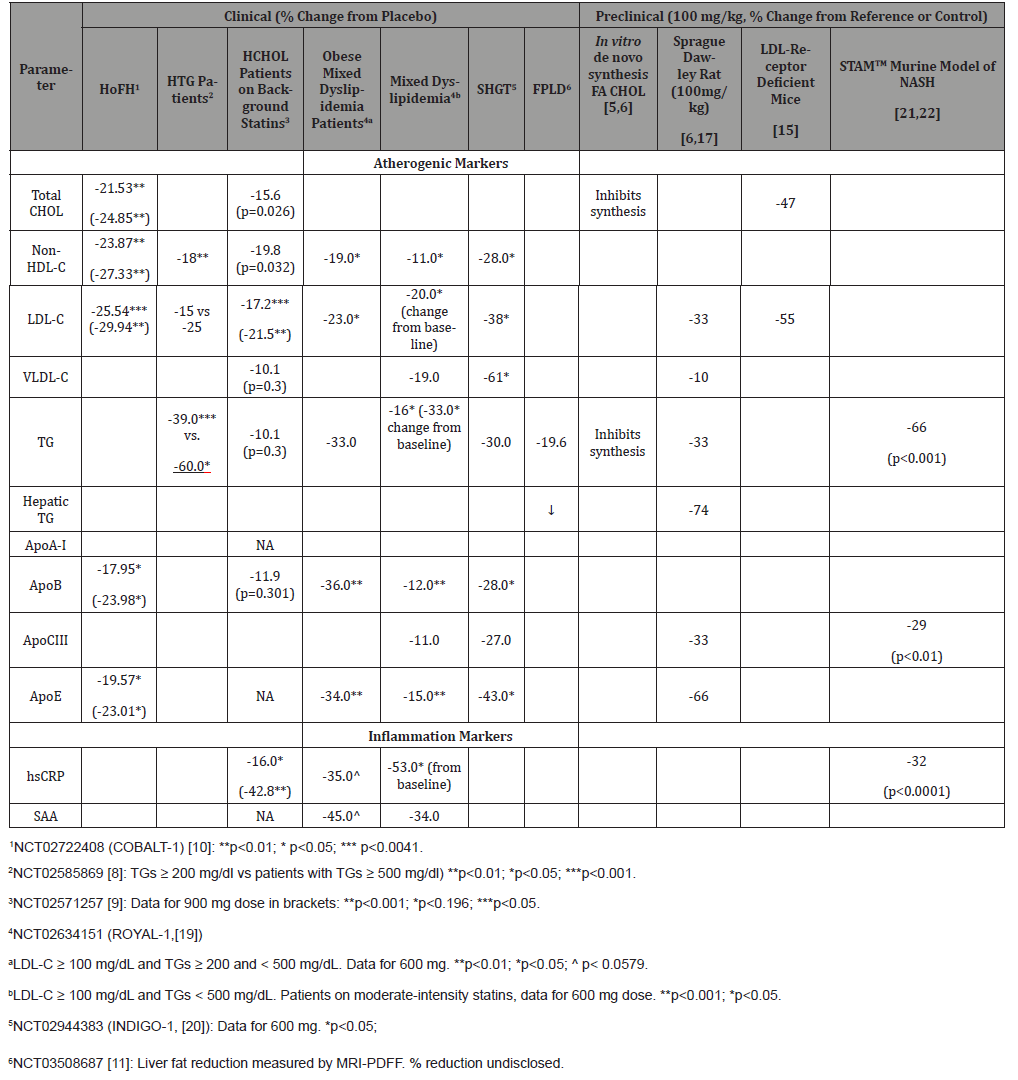
Nonclinical findings with gemcabene translated to clinical results in dyslipidemia. (Table 1) displays meaningful markers that are shared by dyslipidemia, cardiovascular risk and liver disease. Randomized clinical trials with gemcabene administered alone or in combination with statins showed decreases in TG and CHOL in dyslipidemia patients as well as important CRP reductions [9]. Dramatic TG reductions were revealed in hypertriglyceridemia patients: in an ad-hoc analysis of patients with TGs ≥ 200 mg/dl treated with 300 mg gemcabene for 12 weeks, the median percent reduction from baseline was 39%; for a subset of patients with TGs ≥ 500 mg/dl, the median percent reduction from baseline was 60% [8]. In patients with mixed dyslipidemia, recent randomized clinical studies showed that plasma TRIGs, CRP and plasma markers of dyslipidemia, inflammation and overall cardiovascular risk were significantly reduced following treatment with gemcabene [19,20].
Subsets of obese patients with mixed dyslipidemia and patients with SHTG showed an even more dramatic reductions in these parameters. In a double blind, placebo-controlled, Phase 2b trial in patients with mixed dyslipidemia (LDL-C ≥ 100 mg/ dL and TGs ≥ 200 and < 500 mg/dL) treated with gemcabene as an add-on to statins, a pre-defined analysis performed in obese patients (ten treated with 600 mg gemcabene and 8 with placebo, 50% women, with baseline means LDL-C of 142 mg/dL, TGs of 247 mg/dL and BMI of 34 kgm2) showed an overall reduction of the cardiovascular risk markers in this population. 19 Significant reduction of cardiovascular risk markers was also observed in a 12-week randomized, double-blind, placebo-controlled Phase 2b trial in patients with mixed dyslipidemia (LDL-C ≥ 100 mg/dL and triglycerides ≥ 200 and < 500 mg/dL) [20]. Patients treated with 600 mg gemcabene showed a significant reduction in plasma TGs of 47% compared to 27% in placebo-treated patients. In the same study, an analysis of an SHGT patient subset (baseline serum TGs ≥500 mg/dL) treated with 600 mg gemcabene showed a significant reduction in plasma TGs by 30%, and a dramatical decrease in plasma levels of proatherogenic and inflammation markers.
Finally, reduction in liver fat following treatment with gemcabene was evidenced in certain genotypes of FPLD. In a 12- week, open label, proof-of-concept study in FPLD patients, the liver fat fraction measured by MRI-PDFF was reduced in 2 of the 3 responding patients, along with decreases in serum TGs 11, in agreement with observations in preclinical models [6, 17].
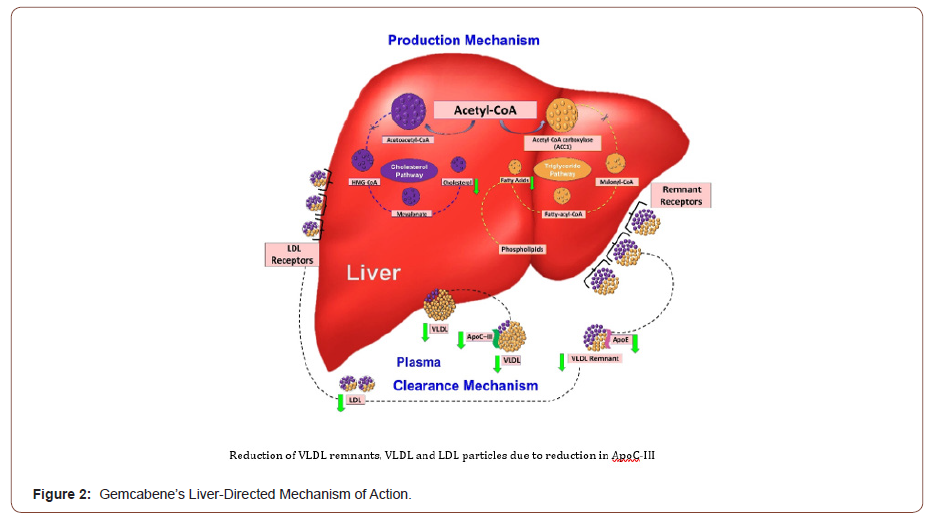
In summary, nonclinical and clinical findings concur in supporting gemcabene’s pleiotropic, liver-directed, mechanism of action, which combines
1) Decrease in lipid production by blocking the overall hepatic production of triglyceride and cholesterol synthesis,
2) Increase in lipid clearance by enhancing the clearance of very-low-density lipoprotein (VLDL),
3) Decrease in liver fat evidenced in diabetic, obese rats and some human genotypes,
4) Decrease in inflammatory markers highly sensitive CRP (hsCRP) and serum amyloid A (SAA) (Figure 2).
Translational Research with Gemcabene: from Dyslipidemia to NAFLD/NASH
Dyslipidemia and particularly obese and HSTG patients are at high risk for NAFLD/NASH, since they often present high plasma TGs, low HDL levels, liver TG, and have a proatherogenic profile. Earlier reports have shown that improvements in NASH result from combining lipid-lowering and anti-inflammatory agents. Gemcabene has shown positive effects in populations at risk of NASH: in obese patients and an SHGT population with TGs over 500 mg/dL, TG plasma levels were decreased, and proatherogenic markers ApoB, ApoC-III and VLDL-C, and markers of inflammation hsCRP and SAA were reduced. Therefore, going back to a nonclinical proof of concept for the NASH indication, gemcabene was tested in the STAMTM diabetic murine model of NASH, as a nonclinical support for translating into clinical trials [21].
In this model, gemcabene’s reductions of TG and CRP are similar to those evidenced by clinical trials in SHGT patients [21] (Table 1). Its effects on the liver histology are relevant to the regression of the disease: decrease in fibrosis with about 40% and significant reduction in the NAS score compared with the vehicletreated NASH, at daily doses translated into clinical doses shown to be effective in SHTG patients [21].
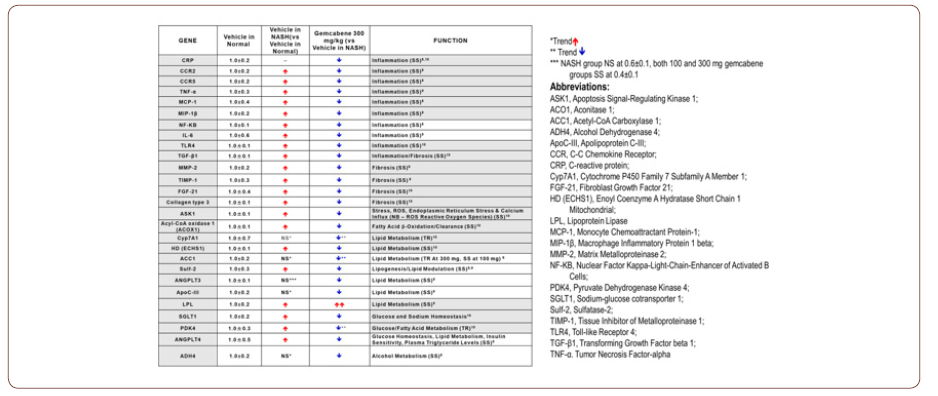
Measurements of hepatic mRNA gene expression (Table 2) are indicative of favorable effects on inflammation, fibrosis, and lipid metabolism at both 100 and 300 mg/kg doses, translatable to clinically effective doses of 300 and 600 mg. In this murine model of NASH, gemcabene significantly downregulated hepatic genes associated with inflammation (IL-6, CXCL1/KC, CRP, TNF-α, MCP-1, MIP-1β, CCR5, CCR2, NF-κB), fibrosis (FGF-21, ASK1, collagen type 3, TLR4, TIMP-1), and lipid and glucose trafficking (apoCIII, Sulf-2, ANGPTL3, ANGPTL4, LPL and SGLT1).
Gemcabene significantly reduced ApoC-III mRNA expression levels, similarly to the plasma ApoC-III reductions in SHTG patients, while decreases in plasma TG and CRP are well correlated with downregulation of the corresponding hepatic genes [18, 21]. Also, gemcabene favorably alters plasma and hepatic gene markers of lipid homeostasis and inflammation, confirming that gemcabene’s pleiotropic effects discovered in dyslipidemia and SHGT nonclinical models and in clinical trials in humans are translated to a nonclinical model of NASH [22].
Table 2: Gemcabene Hepatic Gene Expression in STAMTM Mice Indicative of Favorable Inflammation, Fibrosis and Lipid Metabolism Effects.
Conclusion
In conclusion, findings in clinical trials in SHTG patients and subsets of obese and diabetic subject along with nonclinical data render confidence for further evaluation of gemcabene not only as a treatment for severe hypertriglyceridemia and mixed dyslipidemia, but also as a potential treatment for NASH.
Acknowledgement
None.
Conflict of Interest
No Conflicts of Interest.
References
- Sumida Y, Yoneda M, Ogawa Y, Okanoue T, Nakajima A, et al. (2020) Current and new pharmacotherapy options for non-alcoholic steatohepatitis. Expert Opin Pharmacother 21(8): 953-967.
- Hegele RA, Tsimikas S (2019) Lipid Lowering Agents. Circ Res 124(3): 386-404.
- Wójcik C (2020) Emerging lipid lowering agents targeting LDL cholesterol. Postgrad Med doi: 10.1080/00325481.2020.1751422.
- Tomlinson B, Chan P, Zhang Y, Liu Z, Lam CWK (2020) Pharmacokinetics of current and emerging treatments for hypercholesterolemia. Expert Opin Drug Metab Toxicol 16(5): 371-385.
- Bisgaier C, Creger P, Saltiel A, Tafuri S (1997) Carboxyalkylethers, Formulations, and Treatment of Vascular Diseases. US patent 5,648,387.
- Bisgaier CL, Essenburg AD, Barnett BC, BJ Auerbach, S Haubenwallner, et al. (1998) A novel compound that elevates high density lipoprotein and activates the peroxisome proliferator activated receptor. J Lipid Res 39(1): 17-30.
- Bisgaier CL, Newton RS (2013) Statin-Carboxyalkylether Combinations. US patent 8,557,835.
- Bays HE, McKenney JM, Dujovne CA, Helmut G Schrott, Michael J Zema, et al. (2003) Effectiveness and tolerability of a new lipid-altering agent, gemcabene, in patients with low levels of high-density lipoprotein cholesterol. Am J Cardiol 92(5): 538-543.
- Stein E, Bays H, Koren M, Bakker Arkema R, Bisgaier C (2016) Efficacy and safety of gemcabene as add-on to stable statin therapy in hypercholesterolemic patients. J Clin Lipidol 10(5): 1212-1222.
- Gaudet D, Durst R, Lepor N, Rebecca Bakker Arkema, Charles Bisgaier, et al. (2019) Usefulness of Gemcabene in Homozygous Familial Hypercholesterolemia (from COBALT-1). Am J Cardiol 124(12): 1876-1880.
- (2019) Gemphire Announces Top-Line Data from Familial Partial Lipodystrophy (FPLD) Phase 2a Proof-of-Concept NAFLD/NASH Clinical Trial. Gemphire Press, USA.
- Srivastava RAK, Cornicelli J, Markham B, Bisgaier C (2018) Lipid-lowering Agent Gemcabene Down-Regulates Acute Phase C-reactive Protein via C/EBP-δ-mediated Transcriptional Mechanism. Molecular and Cellular Biochemistry 449: 167-183.
- Srivastava RAK, Cornicelli JA, Markham B, Bisgaier CL (2018) Gemcabene, a First-in-Class Hypolipidemic Small Molecule in Clinical Development, Attenuates Osteoarthritis and Pain in Animal Models of Arthritis and Pain. Front Pharmacol 9: 471.
- Bisgaier CL, Oniciu DC, Srivastava RAK (2018) Comparative Evaluation of Gemcabene and Peroxisome Proliferator-Activated Receptor Ligands in Transcriptional Assays of Peroxisome Proliferator-Activated Receptors: Implication for the Treatment of Hyperlipidemia and Cardiovascular Disease. J Cardiovasc Pharmacol 72(1): 3-10.
- Bisgaier CL, Auerbach BJ (2015) Gemcabene and Atorvastatin Alone and Combined Markedly Reduce LDL-C in LDL Receptor-deficient Mice, a Model of Homozygous Familial Hypercholesterolemia. Circulation 132: A17824.
- Oniciu DC, Bisgaier CB (2017) In Vitro Models Concur with Clinical Results to Confirm Pleiotropic Mechanisms of Action for Gemcabene. Arteriosclerosis, Thrombosis and Vascular Biology 37: A579.
- Bisgaier C, Oniciu DC, Williams JK (2017) An Orally Administered Small Molecule that Inhibits Hepatic Sulfatase-2 Expression in Vivo: a Novel Strategy to Correct Diabetic Dyslipoproteinemia with Implications for Residual Atherosclerotic Cardiovascular Disease (ASCVD) Risk. Circulation 136: A19177.
- Oniciu DC, Srivastava RA, Bisgaier C (2018) Gemcabene Regulates VLDL-Remnant Trafficking and Inflammation Genes with Potential Impact on Cardiovascular Disease. Atherosclerosis 232: 129-130.
- Frias J, Kastelein J, Bakker Arkema R, Liz Masson, Lee Golden, et al. (2017) Gemcabene Add-on Therapy to High- and Moderate-Intensity Statin Stratums in Hypercholesterolemic Patients (ROYAL-1, a Phase 2b Study). Circulation 136: A23082.
- (2018) A 12-Week, Phase 2 Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy Safety and Tolerability of Gemcabene in Subjects with Severe Hypertriglyceridemia (INDIGO-1). Gemphire Press, USA.
- Oniciu DC, Hashiguchi T, Shibazaki Y, Bisgaier CL (2018) Gemcabene downregulates inflammatory, lipid-altering and cell-signaling genes in the STAM model of NASH. PLoS One. 2018;13(5): e0194568.
- Oniciu DC, Srivastava RA, Bisgaier CB (2018) Gemcabene Regulates Hepatic Genes Associated with Inflammation and Fibrosis with Impact on Non-Alcoholic Fatty Liver Disease. Hepatology 68(S1): 1024A.
-
Daniela Carmen Oniciu, Jennifer Lynn Myers. Gemcabene as a Potential Therapeutic for NASH: Lessons Learnt from its Clinical Trials in Dyslipidemia. 2(4): 2020. ACCS.MS.ID.000542.
-
Dialysis Access Steal Syndrome, Ischemic monomelic neuropathy, Limb Ischemia, AV access, Peripheral vascular disease, Paresthesia, Absent pulses, Cerebrovascular disease, Hypertension.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






