 Research Article
Research Article
Experience of Applying Magnetite Nanoparticles in the Treatment of Multiple Sclerosis
A. N. Belousov1,2*, E. Yu. Belousova1 and Ye. V. Lekomtseva3
1Laboratory of Applied Nanotechnology of Belousov, Ukraine
2Kharkiv National Medical University, Ukraine
3State Institution “Institute of Neurology, Psychiatry and Narcology of NAMS of Ukraine”, Kharkiv
A.N. Belousov, Kharkiv National Medical University, Ukraine.
Received Date: March 14, 2023; Published Date: March 27, 2023
Abstract
Multiple sclerosis (MS) is a serious neurological problem because of its high prevalence, chronic course, frequent disability, and propensity to affect young people. The immunopathogenesis hypothesis underlies the origin of MS. Selective sorption activity of biocompatible magnetite nanoparticles against surface proteins of cell membranes, circulating immune complexes, lymphocytotoxic antibodies, complement system, the effect of increasing phagocytic activity and leukocyte phagocytosis completion index allows the effective use of these nanodevices for immunocorrection. The main goal of the study is to slow down the progression of MS, improve the neurological status and general condition of the patient, and reduce the dynamics of the spread of demyelinating foci in the brain. Materials and methods: a patient diagnosed with multiple sclerosis, secondary progressive type of course, cerebro-spinal form, clinical aggravation stage; EDSS neurological status and disability assessment scales; contrast-enhanced MRI of the brain. An oral form of the nanodevice Micromage-B was used as an immunosorbent and immunomodulator. The choice of the regimen and dosage of Micromage-B was personalized. Assessment of the general condition and neurological status was performed every 7 days for 6 months.
Contrast-enhanced MRI of the brain was performed at the 5th month of the study. As a result of using Micromage-B in MS treatment, objective improvement of neurological status, reduction of stiffness and rapid fatigability of the lower extremities were observed. Gait and coordination improved, hand tremors decreased, depression and signs of concentration disorders disappeared, appetite restored, and speech improved. During the entire period of Micromage-B application, positive dynamics in the normalization of the neurological status was observed. After 6 months of treatment, the total score dropped by 165 to 35. It was found that the maximum positive effect was observed in the evaluation of the pyramidal system and coordination. The EDSS Disability Scale score decreased from 6.0 to 5.0. Contrast-enhanced MRI brain examination for the first time showed a decrease in the number of new foci of demyelination in the brain by the 4th month of Micromage-B administration. The positive dynamics of normalization of the neurological status correlated with the results of brain MRI. The process of recovery of central nervous system activity in MS is not only due to the immunosuppressive properties of magnetite nanoparticles, but is probably caused by the activation of remyelination mechanisms and oligodendrocyte differentiation through enzymatic methylation. Considering the above, the use of biocompatible nanodevices in the complex treatment of MS is a promising direction. The scheme and method of using biocompatible magnetite nanoparticles to improve the effectiveness of MS treatment require further improvement and study.
Keywords: Multiple sclerosis; Treatment; Nanodevice; Micromagе-B; Neurological status assessment; Remyelination
Introduction
Multiple sclerosis (MS) is a serious neurological problem due to its widespread occurrence, chronic course, frequent disability of patients and propensity to affect young people. The average age of onset is 30 years. The main hypothesis of immunopathogenesis of MS is that immunological tolerance is violated and autoreactive cells, sensitized to antigens of nervous tissue, actively penetrate through the blood-brain barrier into the brain tissue. B-lymphocytes recognize myelin and order T cells to launch an immune attack [1-7]. T- and B-cells secrete chemicals that attract other immune cells that cause inflammation [8, 9]. Plasma cells produce and release antibodies that attack myelin and enlist the help of other immune cells [10, 11]. T- and B-cells create a permanent residence in the central nervous system (CNS) and continue the attack [12, 13]. There are two hypotheses of MS pathogenesis: the outsidein hypothesis, based on the penetration into the brain tissue of immunocompetent cells activated in the periphery, and inside-out, when the primary is damage to the nervous tissue, the expression of damage receptors - from the family DAMPs (Danger-associated Molecular Patterns), resulting in activation of immunity and failure of tolerance to myelin antigens [14]. Antibodies, which are actively produced by plasma cells, destroy the protective covering of nerve cells. Inflammation develops in the damaged area. After some time the tissue is scarred, which leads to a disturbance of conduction. As a result of such processes, impulses from the brain do not reach the limbs and organs. Because of this a person loses the ability to control his body [15]. The most common initial clinical symptoms are weakness and impaired sensation in one or more limbs, decreased vision, impaired urination and cerebellar ataxia.
Currently, immunomodulatory and immunosuppressive drugs that modify the course of MS form the basis of pathogenetic treatment. Their mechanism of action is related to:
1) Selective or
2) Complete immunosuppression,
3) Prevention of migration of activated cells from lymph nodes or into brain tissue, or
4) A combination of immunoregulatory, anti-inflammatory, antioxidant, and possibly neurotrophic actions.
Possible directions in the development of new treatments for MS include selective local immunocorrection, remyelination and neuroprotection, enhancement of neuroplasticity and relocalization of function, evaluation of the effectiveness and safety of cell therapy, individual selection of therapy based on prediction of pathological process variants and possible response to treatment based on the results of molecular and cellular biology of MS [16-22].
The development of inflammation and demyelination in MS is caused by an impaired immune response, imbalance of T regulatory and T effector cells, activation of B-cell immunity and microglia. All drugs affecting the course of MS either deplete T- or B-cells or alter the signaling pathways associated with the formation of an immune response [23].
Particular attention has recently been paid to the role of B-cells in the chronic inflammatory process in the CNS, as evidenced by their role in both autoantibody formation and in the presentation of antigens and constant activation of T-cells in the brain parenchyma. Anti-B-cell therapy (Rituximab, Ocrelizumab, Ofatumumumab) has proven effective in both remitting and progressive MS. Stimulation of remyelination in MS is associated with the development of new preparations of monoclonal antibodies: anti-Lingo, human immunoglobulin M (IgM). These drugs promote remyelination and differentiation of oligodendrocytes and their precursors [24].
One of the key mechanisms of axonal degeneration is mitochondrial dysfunction. Some drugs (Dimethylfumarate, Idebenone, Biotin) can be considered promising in this direction [25]. The use of drugs affecting the redistribution of ion channels in the demyelinated axon (Lamotrigine, Amiloride, Fampridine) can help to reduce the energy deficit in the axon and neuron [26].
Recently, biocompatible nanotechnological preparations have been increasingly used in medicine. Since 1998, Ukrainian clinics have been officially using nanodevices developed by Belousov’s Applied Nanotechnology Laboratory: Micromage-B, MCS-B and ICNB brands [27]. These nanodevices are based on biocompatible magnetite nanoparticles. The peculiarity of physical and chemical characteristics provides a condition for a wide range of their application in order to influence the quantitative and qualitative compositions of the fluid environment of the body, metabolic and biochemical processes, the energy balance of cells. Selective sorption activity against surface proteins of cell membranes, circulating immune complexes, lymphocytotoxic antibodies, complement system, the effect of increasing phagocytic activity and leukocyte phagocytosis completion index [28] allow the effective use of these nanodevices for immunocorrection. In addition, the nanopreparations presented have an effect on glycolysis processes, the activity of cell membrane ion channels, normalize erythrocyte function, improve microcirculation, reduce platelet aggregation index [29-31], activate the system of antiradical enzymes, and inhibit lipid peroxidation processes [32, 33].
The results of these studies are a prerequisite for the development of innovative techniques for the effective and safe use of biocompatible magnetite nanoparticles in the treatment of severe autoimmune diseases, including multiple sclerosis. The main goal of the study is to slow down the progression of MS, improve the neurological status and general condition of the patient, and reduce the dynamics of the spread of demyelinating foci in the brain.
Materials and Methods
Patient K. was diagnosed with multiple sclerosis, secondary progressive type of course, cerebrospinal form, clinical aggravation stage, pronounced spastic tetraparesis with accent in lower extremities with walking impairment, pronounced urinary-ataxic syndrome, sphincter and sensory disorders. MRI-signs of multifocal diffuse brain lesions (more than 30) of demyelinating nature. Active phase. Diffuse atrophic process in the cerebral cortex. The mean number of relapses during the year before study inclusion was 1.0. The EDSS disability score was 6.0. The duration of the disease since the onset of the first symptoms was 24 years. For 14 years, the patient was regularly treated with vascular and metabolic drugs in combination with hormonal therapy. In the 6th year, against the background of active treatment, the disease transformed into a secondary-progressive type of course. The therapy was supplemented with the immunosuppressive drug Teriflunomide.
However, despite all treatment, the patient’s general condition progressively worsened. The neurological status has not stabilized. MRI examination showed a progressive increase in the number of new foci of demyelination in the brain over the last 4 years.
Considering the above, the complex therapy was supplemented with the prescription of Micromage-B [34]. Micromage-B is an oral form of nanodevice officially registered by the Ministry of Health of Ukraine. Micromage-B is a powder form of magnetite (Fe3O4) nanoparticles, which is designed for the prevention and treatment of various diseases, as well as to increase the body’s resistance to adverse environmental factors. Micromage-B it is device of nanotechnology. The size of magnetite nanoparticles is from 6 to 12nm. The therapeutic effect of the nanopreparation is based on the mechanism of sorption and action of a constant magnetic field on cellular and subcellular structures that causes by magnetite of nanoparticles. The area of the sorption surface of nanoparticles is from 800 to 1200 m2/g, and the value of the induced magnetic field is 300-400 kA/m. The application point of Micromage-B is the microenvironment of the cell aqueous spaces, surface membrane proteins. Magnetite nanoparticles by selective sorption change the quantitative and qualitative composition of cell surface proteins, and the permanent magnetic field induced by magnetite nanoparticles changes the mobility and orientation of hydrogen protons in the water sectors of the cell microenvironment. The latter contributes to the activation of hydrolysis of the phosphate residue ATP.
In general, the transmembrane exchange and metabolism of the cell are modified, and its susceptibility is altered. Nanopreparation causes increase of adaptive mechanisms and potential of cell organelles, accelerates of the reparative processes at the level of membranes and macromolecules. Micromage-B significantly increases the level of synthetic intracellular reactions and compensatory possibilities of the organelles of the cells of the renal glomerulus, the epithelial cells of the proximal and distal sections of the nephron, that is structurally expressed in increasing bioenergetic support of synthetic intracellular processes, increasing the reparative and adaptive capabilities of nephrons. By increasing the activity of redox phosphorylation processes, providing energy requirements of synthetic intracellular reactions proceeding at the level of membranes and macromolecules in liver cells, Micromage-B acts as a direct activator of reparative intracellular processes in hepatocytes, enhances glycogenogenesis. This fact allows to use it effectively as a powerful hepatoprotector in the treatment of acute and chronic liver diseases. Micromage-B can act as an active stimulator of erythropoiesis, thereby quickly restoring the level of hemoglobin in the blood. Micromage-B stimulates the synthesis of pulmonary surfactant, which provides stretchability and elasticity of lung tissue. Micromage-B provides the stability of the lungs to negative influences of factors of external and internal environment by means increasing the activity of alveolar macrophages. Clinically and laboratory: Micromage-B improves microcirculation and rheological properties of blood by stabilizing the bioelectric charge of erythrocyte membranes. It has a pronounced immunomodulatory effect. Micromage-B has a selective bacteriostatic effect in relation to the pathogenic microflora, without adversely affecting the normoflora. Delays the growth and reproduction of various kinds of fungi. Stimulates the growth and activity of lactic acid bacteria in the intestine. In 2-3 times increases the effectiveness of antibacterial and antifungal agents. These facts allow the effective use of Micromage-B for the treatment of dysbacteriosis and candidiasis. Nanoparticles of Micromage-B adsorbed toxic substances and circulating immune complexes.
It’s fact that greatly increases the effectiveness of treatment of various allergic diseases, autoimmune processes (for example, rheumatoid arthritis, acute and chronic polyarthritis, eczema etc.), acute poisoning. Improving the renal blood flow by Micromage-B has a moderate diuretic effect. Having in its nature magnetic moment the magnetite nanoparticles brakes down and promotes the dissolution of stones in the kidneys and bile ducts of the liver, which are subsequently eliminated through the excretory systems of the organism in the form of magnetically sensitive crystals forms. Micromage-B actively contributes to the normalization level of lipids and proteins in blood. Affects the factors of atherogenesis, preventing the development of atherosclerotic processes. Active decline the level of release into the blood of an excessive number of mediators of the aggressive state is result of actions permanent magnetic field of magnetite nanoparticles of the Micromage-B into the macrophage cells. In turn, this causes pronounced antiinflammatory and moderate analgesic effects. Micromage-B modulates activity of the enzyme link of antioxidant system, sorbs products of the lipid peroxidation, corrects balance between antiradical and proradical products. Under the influence of Micromage-B, the monocytes actively synthesized a tumor necrosis factor (cachexin), which has cytotoxic and cytostatic effects on tumor cells. When topical application (in the form of powders, ointments or aqueous solutions of colloids) the Micromage-B promotes rapid wound healing of mucous membranes and skin, change of wet tissue necrosis to dry. Micromage-B does not cause disturbances in the functional activity of organs and body systems, it is non-toxic [35, 36].
Dosing regimen Micromage-B: the first month - 500 mg daily, the second month - 500 mg every other day, subsequent months - 500 mg once every three days. The choice of the scheme and dosage of Micromage-B had an individual approach. The time and speed of the appearance of signs of improvement in the patient’s general state and recovery of neurological status were taken into account.
The dynamics of changes in the neurological status were studied using a modified Multiple Sclerosis Patient Assessment Scale [37, 38]. This scale is based both on the severity of motor disorders and on their combination with other signs of damage to the nervous system. For quantitative assessment of disability, Kurtzke’s online EDSS calculator was used [39]. The manifestations of foci of cerebral demyelination were studied using contrast-enhanced MRI.
The patient’s general condition and neurological status were evaluated every 7 days for 6 months. According to the plan, a contrast-enhanced MRI of the brain was performed once a year. In this case, the timing of the MRI coincided with the 5th month of use of Micromage-B.
Table 1: Neurological status assessment scale for patient K. with multiple sclerosis.
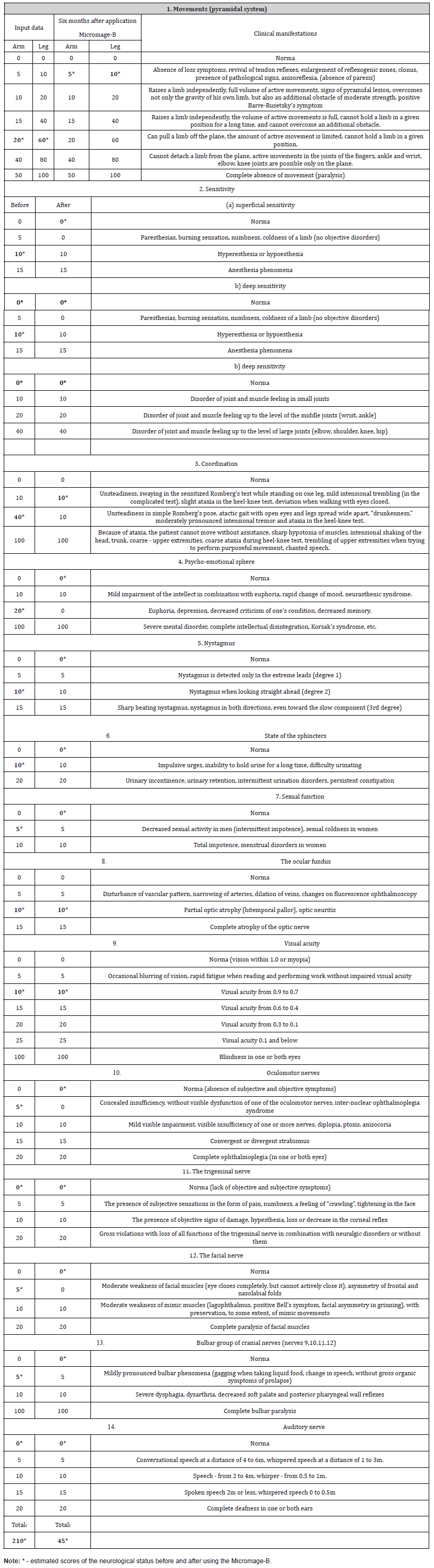
Results
One week after the use of Micromage-B, there was a noticeable improvement in the patient’s neurological status. The patient noted a significant decrease in stiffness and rapid fatigue in the lower extremities. Objectively, the gait and coordination improved, the hand tremor decreased, depression and concentration disorders completely disappeared, appetite restored, and speech improved. Positive dynamics of normalization of the neurological status was constantly observed during the whole period of Micromage-B application. Evaluation of the neurological status of a patient with multiple sclerosis before and after 6 months of Micromage-B is presented in Table 1.
The data in Table 1 demonstrate positive dynamics in the normalization of the neurological status after 6 months of using Micromage-B. At the initial stage the total number of points was 210. After 6 months of using Micromage-B-45. Thus, the total number of points dropped by 165. The maximum positive effect was observed in the evaluation of the pyramidal system and coordination. The EDSS disability scale score decreased from 6.0 to 5.0.
Contrast-enhanced MRI examination of the brain after 4 months of use of Micromage-B showed for the first time a decrease in the number of new foci of demyelination in the brain. The positive dynamics of the neurological status correlated with the results of the MRI examination of the brain.
Analyzing the data obtained, special attention should be paid to the fact that against the background of the predicted positive clinical effect caused by immunosorption, the use of magnetite nanoparticles (Micromage-B) actively contributed to the restoration of neurological status. In this case, it can be caused by the processes of remyelination and differentiation of oligodendrocytes. Oligodendrocytes are a type of neuroglia cells that cover the myelin sheath of neurons in the CNS. The molecular mechanisms underlying cell differentiation and specialization are perhaps the most confusing area of cell biology and developmental biology. The development and maturation of many cell types is still poorly understood.
However, it is now known that one of the mechanisms of oligodendrocyte maturation is the process of enzymatic methylation - the addition of a methyl group (-CH3) to the nitrogen atom N6 in the nitrogenous base of adenosine, known as m6Amethylation. Such a seemingly insignificant change can have enormous consequences for further stages of protein biosynthesis. The role of m6 A-methylation has been shown in many processes related to oligodendrocyte maturation [40]. The universal donor of methyl groups in the body is s-adenosine-methionine, which is formed as a result of the interaction of the amino acid methionine with the ATP molecule.
Considering that due to the activation of glycolysis Micromag-B significantly increases the amount of macroergic compound (ATP) [41, 42], as well as the formation of the reduced form of the coenzyme nicotinamide-adenindinucleotide phosphate (NADPH2) with the transition of oxidized form of glutathione to reduced [43], conditions for starting enzymatic methylation processes are created. The latter most likely complements the mechanism of action of magnetite nanoparticles (Micromage-B). Eventually, it ensures differentiation of oligodendrocytes and the remyelination process. It is also necessary to take into account the fact that these magnetite nanoparticles polarize the aqueous sector of the cellular microenvironment [44]. As a result, hydrolysis of the phosphate residue of ATP is activated, a quantum of energy is reset, and ADP is formed.
Taking into account the positive dynamics of the neurological status, it was decided to continue the use of the nanopreparation Micromage-B according to the specified dosage. The therapy was supplemented with a complex of rehabilitation exercises, aimed at the earliest restoration of physical, cognitive and psychosocial functions of a patient with MS.
Conclusion
The results of the study expanded the range of clinical efficacy of biocompatible magnetic nanoparticles in the therapy of severe autoimmune diseases. The use of nanopreparation Micromage-B in the treatment of MS had a pronounced positive clinical effect on the restoration of neurological status, objectively contributed to a reduction in the number of new foci of demyelination in the brain. The process of recovery of CNS activity in MS is not only due to the immunosuppressive properties of magnetite nanoparticles, but is probably caused by the activation of remyelination mechanisms and oligodendrocyte differentiation through enzymatic methylation. The use of biocompatible nanodevices in the complex treatment of multiple sclerosis is a promising direction. The scheme and method of using biocompatible magnetite nanoparticles to improve the effectiveness of MS treatment require further improvement and study.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Duffy SS (2014) The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int 2014: 285245.
- Ortiz GG (2014) Role of the blood-brain barrier in multiple sclerosis. Archives of Medical Research 45: 687-697.
- Larochelle C (2011) How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Letters 585(23): 3770-3780.
- Cross AH, Waubant E (2011) MS and the B cell controversy. Biochim Biophys Acta 1812(2): 231-238.
- Dalakas MC (2008) B cells as therapeutic targets in autoimmune neurological disorders. Nature Clinical Practice Neurology 4(10): 557-567.
- Constant SL (1999) B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol 162(10): 5695-5703.
- Crawford A (2006) Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol 176(6): 3498-3506.
- Bar Or A (2010) Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS. Ann Neurol 67(4): 452-461.
- Duddy M (2007) Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 178(10): 6092-6099.
- Genain CP (1999) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5(2): 170-175.
- Storch MK (1998) Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol 43(4): 465-471.
- Serafini B (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14(2): 164-174.
- Magliozzi R (2010) A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68(4): 477-493.
- Sarah Dhaiban, Mena Al-Ani, Noha Mousaad Elemam, Mahmood H Al-Aawad, Zeinab Al-Rawi, et al. (2021) Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Sci 3: 12.
- Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J (2017) New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 23: 1123-1136.
- Farooqi N, Gran B, Constantinescu CS (2010) Are current disease-modifying therapeutics in multiple sclerosis justified on the basis of studies in experimental autoimmune encephalomyelitis? Neurochem 115: 829-844.
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. Engl. J. Med 354:899-910.
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, et al. (1992) Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356: 63-66.
- Ridge SC, Sloboda AE, McReynolds RA, Levine S, Oronsky AL, et al. (1985) Suppression of experimental allergic encephalomyelitis by mitoxantrone. J Clin Immunol Immunopathol Res 35: 35-42.
- Huang W, Chen W, Zhang X (2017) Multiple sclerosis: Pathology, diagnosis and treatments. Ther. Med13: 3163-3166.
- Ghasemi N, Razavi S, Nikzad E (2016) Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J 19: 1-10.
- Hanafy KH, Sloane JA (2011) Regulation of remyelination in multiple sclerosis. FEBS Lett 585: 3821-3828.
- Ghasemi N, Razavi S, Nikzad E (2016) Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J 19: 1-10.
- Hoglund RA, Maghazachi AA (2014) Multiple sclerosis and the role of immune cells. World J Exp Med 4: 27-37.
- Isabella Peixoto de Barcelos, Regina M Troxell, Jennifer S Graves (2019) Mitochondrial Dysfunction and Multiple Sclerosis. Biology (Basel) 8(2): 37.
- Mustafa S Hamada, Maarten HP (2015) Myelin Loss and Axonal Ion Channel Adaptations Associated with Gray Matter Neuronal Hyperexcitability. J Neurosci 35(18): 7272-7286.
- http://www.nanolab.com.ua/
- Belousov AN (2011) The use of magnetite nanoparticles in applied medicine. Materials Science Forum 694: 205-208.
- Belousov AN (2014) The role of magnetite nanoparticles (ICNB) in discovery new factor which influence on permeability of erythrocytes and eryptosis. Open Access Library Journal 1: e1055.
- Belousov AN, Malygon E, Yavorskiy V, Belousova E (2018) Application of the stanardized form magnetite nanoparticles (ICNB) in creature simple and practical method of additive modernization of preservation solutions for red blood cells. Global Journal of Anesthesia and Pain Medicine 1(1).
- Belousov A (2020) Study of the Effect of Nanotechnology Drugs (MCS-B) on the Aggregation of Human Blood Platelets. Journal of Nanosciences Research & Reports. Scientize Publishers 1(1): 1-8.
- Belousov A, Malygon E, Yavorskiy V, Belousova E (2019). Innovative method of nanotechnology to increase the storage time of RBCs due by stabilizing the molecular structure of proteins and lipids of erythrocyte membranes. Biomedical Journal of Scientific & Technical Research 13(4): 10079-10087.
- Belousov A, Kalynychenko T, Malygon E, Anoshyna M, Yagovdik M, et al. (2022) Research of Lipid Peroxidation after Administration of Nanomodified Resuspending Solution in Donor Red Blood Cells on During Their Storage. International Journal of Biomed Research 2(2).
- AN Belousov (1998) Patent Agency of Ukraine No. 30538А UA A 23L 1/304 Therapeutic and preventive product MICROMAGE-B / № 98052704: 6-11.
- http://nanolab.com.ua/products/micromagindex.html
- Belousov A (2018) A New Promising Method of Hepatitis Treatment at the Level of Ultrastructure of the Liver by Standardized Powder form of Magnetite Nanoparticles (Micromage-B). Journal of Pharmacology & Clinical Research 6(1): 555676.
- Gordeev YY, Shamova TM, Semashko VV (2006) Scale of evaluation of the neurological status in multiple sclerosis. Journal of GrSMU 1:75-78.
- Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11): 1444-1452.
- https://edss.neurol.ru/
- Huan Xu, Yulia Dzhashiashvili, Ankeeta Shah, Rejani B Kunjamma, Yi-Lan Weng (2020). m6A mRNA Methylation Is Essential for Oligodendrocyte Maturation and CNS Myelination. Neuron 105(2): 293-309.e5.
- Belousov A (2012) Effect on Hemolysis and Transport ATPase Activity of Erythrocytes by Means Nanopareticles of Magnetit Controlled Sorbent (MCS-B). Pain, Anesthesia and Intensive Care 1: 26-28.
- Belousov A, Belousova E (2014) The Role of Magnetite Nanoparticles (ICNB) in Discovery New Factor Which Influence on Permeability of Erythrocytes and Eryptosis. OALib Journal is an all-in-one open access journal 1: 1-6.
- Belousov A, Kalynychenko T, Malygon E, Anoshyna M, Yagovdik, M, et al. (2021) Study of effects a new resuspending solution which was nanotechnologically upgraded on lipoperoxidation, catalase activity and red blood cell peroxidation resistance in donor blood components. Archives in Biomedical Engineering & Biotechnology 6(2): 1-6.
- Belousov AN(2013) Myth and reality application of magnetite nanoparticles as selective contrasting means of the malignant tumors in MRI investigation. Biomedical Engineering Research 2(3): 147-152.
-
A. N. Belousov*, E. Yu. Belousova and Ye. V. Lekomtseva. Experience of Applying Magnetite Nanoparticles in the Treatment of Multiple Sclerosis. Arch Clin Case Stud. 3(3): 2023. ACCS.MS.ID.000564.
-
Chronic course, Lymphocytotoxic antibodies, Neurological status, Magnetite nanoparticles, Central nervous system, Danger-associated Molecular Patterns, Mitochondrial dysfunction, Urinary-ataxic syndrome, Allergic diseases.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






