 Case Series
Case Series
Effects of Metformin on Individuals with Fragile X Syndrome and Additional Genetic Variants
Van Kim Ma1,2*, Ramkumar Aishworiya2, Elysa J Marco3 and Randi Hagerman1,2
1*Department of Pediatrics, University of California, Davis, California, United States
2MIND Institute, University of California, Davis, California, United States
3Neurodevelopmental Medicine, Cortica Healthcare, California, United States
Van Kim Ma, Department of Pediatrics, University of California, Davis, California, United States
Received Date: October 26, 2024; Published Date: November 12, 2024
Abstract
Background/Objectives: Fragile X syndrome (FXS) is caused by more than 200 CGG repeats in the promoter region of the fragile X messenger ribonucleoprotein (FMR1) located at Xq27.3. While clinical characteristics may vary, most individuals have intellectual disability, speech/language delays, and behavioral disturbances including social anxiety, inattention, hyperactivity, impulsiveness, and aggression. The presence of additional genetic variants is associated with a more severe phenotype in individuals with FXS. Metformin is being studied as a potential targeted treatment for individuals with FXS with studies showing improvements in language and behavior after metformin treatment. This is the first report on the positive benefits of metformin in individuals with FXS and additional genetic variants.
Methods: Three individuals with FXS and additional genetic variants, with associated speech and language delay and behavioral concerns were trialed on metformin to evaluate for behavioral change. Clinical Global Impression, Global Improvement (CGI-I) and the Aberrant Behavior Checklist- Community Edition (ABC-C) were obtained in retrospect after starting metformin.
Results: All three individuals demonstrated much improvement on the CGI-I. Two individuals demonstrated a decrease in irritability, social irresponsiveness, stereotypy, hyperactivity, inappropriate speech, and social avoidance.
Conclusions: Metformin has been shown to improve speech and language and cognitive abilities and decrease externalizing behaviors in individuals with FXS. Individuals with FXS and additional genetic variants may benefit from the use of metformin as well.
Keywords: Genetic variants; fragile X syndrome; autism spectrum disorder; strabismus
Introduction
Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability and monogenic cause of autism spectrum disorder (ASD) [1]. It is a neurodevelopmental disorder caused by excessive CGG repeats in the promoter region of the fragile X messenger ribonucleoprotein gene (FMR1) located at Xq27.3. The presence of 200 or more CGG repeats in the 5’ non-coding region of the FMR1 gene results in an increase in methylation of the FMR1 promoter and thus silencing gene expression, leading to the reduction or absence of fragile X mental retardation protein (FMRP) [2,3]. The level of FMRP is correlated with symptom severity; individuals with mild to moderately low levels of FMRP generally show less severe symptoms than those with extremely low levels [4]. The correlation between FMRP level and a diagnosis of ASD have also been observed [5].
The clinical presentation of FXS varies and features, with the exception of hypotonia, may not be present at birth. The classic physical features of FXS include large prominent ears, long face, hyperflexible finger joints, strabismus, connective tissue related problems, double-jointed thumbs, and macroorchidism. In addition, individuals with FXS typically have developmental delays and neurobehavioral challenges. Delays can be in speech and language development, motor skills, and in cognitive development. Most individuals have behavioral disturbances such as social anxiety, inattention, hyperactivity, impulsivity, aggression, repetitive behaviors, and obsessive and compulsive tendencies [1,6,7]. FXS is also associated with increased occurrence of epilepsy, recurrent ear infections, and reflux in the neonatal period [8,9]. Intellectual disability is present in most males with FXS. However, individuals of female sex tend to have a less severe phenotype compared with individuals of male sex, as females typically have a normal X chromosome in addition to the X chromosome with the full mutation, thus producing more FMRP than males with FXS. Hence, about 30% of females with FXS have an intelligence quotient (IQ) within normal limits, 30% have a borderline IQ (70 to 85) and 30% have an IQ below 70, but most may still have challenges with inattention, social anxiety, and speech and language deficits [10,11]. Genetic testing identifies 99% of FXS cases but some individuals with FXS may also have additional genetic variants that can significantly impact their clinical presentation and management [12].
Advances in the understanding of the pathophysiology of FXS has led to the study of various targeted treatments including metabotropic glutamate receptor 5 antagonists, γ-aminobutyric acid modulators, minocycline, selective serotonin reuptake inhibitors, cannabidiol (CBD), phosphodiesterase inhibitors (PDE4D) and most recently, metformin [13-17]. Drosophila and knock out mouse models of FXS have found that metformin improves memory, social novelty deficits, and neuroanatomical abnormalities [18-20]. Preliminary evidence shows that metformin can be used as a targeted treatment for cognitive and behavioral problems associated with FXS [21,22]. Case series have shown that children and adults with FXS treated with metformin demonstrated improvements in irritability, hyperactivity, social responsiveness, and language [22,23]. One case report has also shown a relative maintenance and mild elevation of IQ in two adults with FXS treated with metformin as compared to the typical decline in IQ over time reported in most adults with FXS [23].
We present three cases of individuals who have FXS and at least one additional genetic variant who have all demonstrated benefit from oral metformin medication treatment. Benefits of metformin in these individuals, two children and one young adult, included improvement in language and behaviors, particularly in emotional regulation.
Materials and Methods
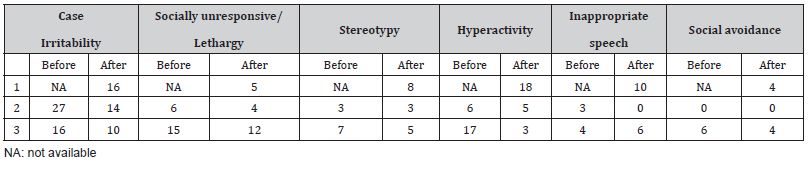
Individuals with FXS and additional genetic variants were seen at a tertiary academic center by an established clinician in the field. All individuals with speech/language delay and behavioral concerns were trialed on metformin to evaluate for clinical change. Both the before and after Clinical Global Impression, Improvement (CGI-I) and the Aberrant Behavior Checklist-Community Edition (ABC-C) were obtained in retrospect after starting metformin (Tables 1&2). The ABC-C is a 58-item questionnaire completed by caregivers to assess the presence and severity of various behaviors commonly seen in individuals with neurodevelopmental disabilities. Results of the ABC-C are reported based on the modified subscales of the ABC-C specific for FXS [24]. This uses 54-questions from the ABC-C to generate 6 subscales; namely irritability, lethargy, social avoidance, stereotypic behavior, hyperactivity, and inappropriate speech. Higher scores represent greater challenging behaviors. Caregivers rated behaviors on a scale of 0 to 3 with 0 representing not a problem at all, 1 representing slightly a problem, 2 being moderately a problem, and 3 being severe in degree.
Table 1: Summary of cases including genetic variants, final dose of metformin, duration of metformin to noticeable benefits, other medications taken, and CGI-I.
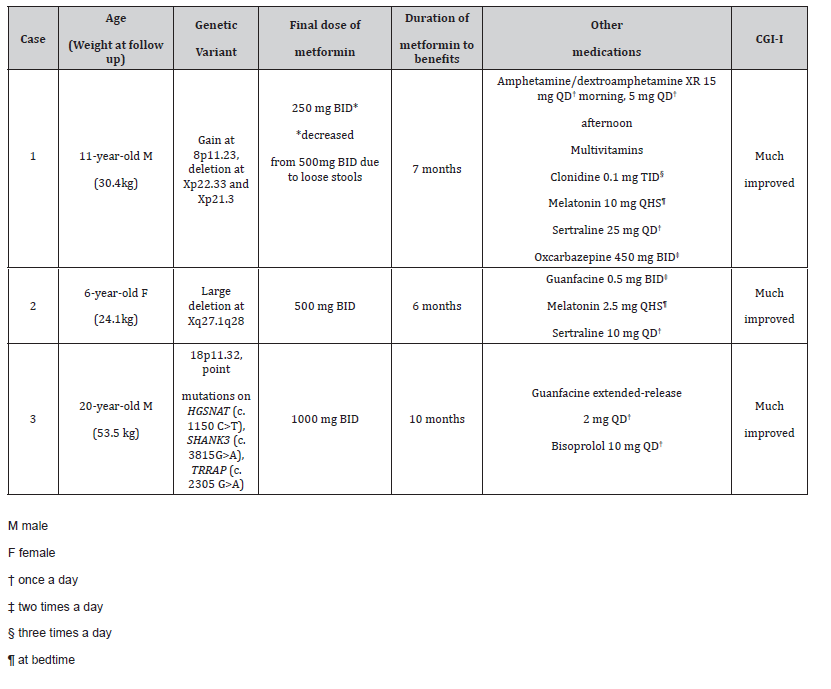
Table 2: Results of the ABC-C before and after starting metformin.
Case Reports
Case 1
11-year-old boy with FXS and gain at 8p11.23, and deletions at Xp22.33 and Xp21.3. At the initial evaluation, he was reported to be speaking in short phrases and exhibiting significant daily outbursts in school and at home. He was also very aggressive toward caregivers. He was started on metformin 500 mg once daily. At the follow-up visit seven months after starting metformin, he was reported to be speaking in 5–7-word sentences, able to communicate his desires, have back and forth conversations for two turns, and follow multi-step directions. Caregivers also noticed a substantial decrease in outbursts and aggression. Caregivers reported receiving positive reports from school for the first time. ABC-C data from prior to starting metformin is not available as he was living with different caregivers at the time. However, ABC-C data after starting metformin showed scores were mostly rated as not a problem at all and slightly a problem. The hyperactivity and inappropriate speech subgroups were most notable for behaviors that were rated as a severe problem. Overall, caregivers noted that his behaviors and skills were much improved with metformin. His dose of oxcarbazepine increased during the follow up period but there was no change in other medications.
Case 2
6-year-old girl with FXS related to a large deletion at chromosome X (q27.1q28) that resulted in loss of multiple genes in addition to FMR1 gene. At baseline, she spoke in short phrases and was easily frustrated. She was started on metformin 400 mg twice a day. Six months after starting metformin, she was noted to have significant gains in language skills; she was speaking in complete sentences and had improvements in language comprehension. Caregivers also reported improvements in emotional regulation skills and fewer tantrums. The ABC-C data primarily showed notable improvements in irritability and inappropriate speech with a slight decrease in social irresponsiveness and hyperactivity. Overall, caregivers noted that her behaviors and skills were much improved after starting metformin. There were no changes in her medications or therapies during the follow-up period.
Case 3
20-year-old male with FXS and loss at 18p11.32. Prior to starting metformin, he struggled with receptive and expressive language. His speech was limited to 1–2-word phrases. He was started on metformin 500 mg once daily and gradually titrated to 1000 mg twice a day over 10 months. At the follow up visit ten months later, he had improvements in language, and decreased anxiety and impulsivity. He was also more aware of his surroundings and of others around him. Thirty months later, he was speaking in 3–6- word phrases and reading sight words. He also had improvements in attention and was able to attend activities for up to 90 minutes in class. Results from the ABC-C data showed a remarkable reduction in irritability and hyperactivity. Some improvements in social unresponsiveness, social avoidance, and stereotypy were also noted. There was an increase in appropriate language, but this may be an unintended result of improvement in language. Overall, caregivers rated his behaviors and skills as much improved with metformin.
For all three individuals, metformin was not started simultaneously with other medications and dose changes to other medications were made only after metformin was started so caregivers were able to monitor potential benefits and/or side effects of each individual medication. All participants were previously enrolled in non-pharmacological therapies prior to starting metformin and no additional therapies were started.
Discussion
Metformin is a biguanide oral anti-hyperglycemic medication that has been well-established as first-line therapy for adults with type 2 diabetes and in adults with prediabetes to prevent progression to diabetes [25]. Metformin is typically used in conjunction with life-style modifications in these adults. It has also been established as a safe and effective treatment of type 2 diabetes in the pediatric population at doses of up to 1,000 mg twice a day [26]. Metformin is typically well tolerated although common side effects include gastrointestinal symptoms such as diarrhea and abdominal pain [25]. One individual (case 1) in our cohort required a reduction in dose due to significant loose stools.
Since its approval by the Food and Drug Administration in 1994, metformin has been studied and continues to be studied for its effect on other health outcomes and diseases. It has been found to be an effective drug in promoting weight loss in insulin sensitive and insulin resistant overweight and obese individuals [27] and has been investigated as an anti-aging treatment [28].
In addition, animal studies in FXS have demonstrated an overactive insulin receptor and up-regulation of the mammalian target of rapamycin complex 1 and mitogen activated protein kinase/extracellular signal-related kinases signaling pathways, as well as elevation of matrix metalloproteinase-9 levels in the absence of FMRP, the protein produced by the FMR1 gene [18,20,29]. Metformin has been shown to act as a strong inhibitor of the mammalian target of rapamycin pathway and improve cognitive and social behaviors [11-19,30]. It has been shown to improve irritability, aggression, and social avoidance in individuals with FXS and anecdotal clinical evidence has suggested improvements in language and cognitive skills [15].
It is possible that metformin is especially helpful in individuals with FXS and additional genetic variants who are especially atrisk for cognitive and behavioral impairments. It may be that the beneficial effects seen in the individuals in this case series was due to their ongoing other therapies and medications apart from metformin alone. A placebo-controlled randomized controlled trial will be important to conclusively determine the effects of metformin; nonetheless this case series provides preliminary data about the potential beneficial effects of metformin. None of the individuals had any major side effects after starting the metformin. Further, metformin is a well-established drug that has been in use for many decades with a good safety profile. Thus, a trial of metformin in conjunction with other treatments including behavioral intervention may be warranted in individuals with FXS and additional genetic variants. It is also important to consider further genetic testing in individuals with FXS who have a more severe clinical presentation than expected to identify other underlying genetic variants if any. This can be useful in prognostication for these individuals as well as for better medical management of associated comorbidities related to the genetic variant.
A limitation of this study is that the ABC-C questionnaires were completed several months after the patients were started on metformin. Some caregivers had difficulty recalling behaviors before starting metformin and thus ratings on the ABC-C may not be an accurate representation of the improvements seen. Also, one individual was not in the care of the current caregivers before starting metformin and was not able to accurately complete the ABC-C. An additional limitation is that all three individuals were also on several other medications at the time same and participating in non-pharmaceutical therapies (although no changes to nonpharmaceutical therapies during the follow up period). It is possible that a combination of metformin, other behavioral medications, and non-pharmaceutical therapies contributed to the improvements described.
Conclusion
Individuals with FXS may have other unidentified genetic variants; testing to identify for possible genetic variants is recommended in individuals with an atypical clinical presentation. Metformin has been shown to improve speech and language and cognitive abilities, and decrease behaviors such as irritability, aggression, and social avoidance in individuals with FXS. Individuals with FXS and additional genetic variants can have improvements in language and cognitive abilities, and behavioral disturbances with metformin as well. However, there are several limitations of this case series including the manner in which the ABC-C data was collected, the CGI-I data not rated in conjunction with the Clinical Global Impression-Severity, and the impact of other medications and nonpharmaceutical therapies is unclear.
Acknowledgement
We would like to acknowledge the families who participated in this case series. This work was partially funded by the Azrieli Foundation, the Victor LaFave lll Fund, and the MIND Institute IDDRC grant P50 HD103526.
Conflict of Interest
RH has received funding from Zynerba/Harmony Pharma and Tetra/Shionogi Pharma to carry out trials in children and adults with fragile X syndrome at UC Davis Health.
References
- Hagerman RJ, Hagerman PJ (2008) Testing for fragile X gene mutations throughout the life span [published correction appears in JAMA. 2009 Feb 11;301(6): 602]. JAMA 300(20): 2419-2421.
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, et al. (1992) DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet 1(6): 397-400.
- Saldarriaga W, Tassone F, González-Teshima LY, Forero-Forero JV, Ayala-Zapata S, et al. (2014) Fragile X syndrome. Colomb Med (Cali) 45(4): 190-198.
- Kim K, Hessl D, Randol JL, Espinal GM, Schneider A, et al. (2019) Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat ex-pansions. PLoS One 4(12): e0226811.
- Budimirovic DB, Schlageter A, Filipovic-Sadic S, Protic DD, Bram E, et al. (2020) A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments. Brain Sci 10(10): 694.
- Bailey DB Jr, Raspa M, Olmsted M, Holiday DB (2008) Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A146A(16): 2060-2069.
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, et al. (2017) autism spectrum disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 139(Suppl 3): S194-S206.
- Berry-Kravis E, Filipink RA, Frye RE, Golla S, Morris SM, et al. (2021) Seizures in Fragile X Syndrome: Associations and Longitudinal Analysis of a Large Clinic-Based Cohort. Front Pediatr 9: 736255.
- Baker C, Retzik-Stahr C, Singh V, Plomondon R, Anderson V, et al. (2021) Should metformin remain the first-line therapy for treatment of type 2 diabetes?. Ther Adv Endocrinol Metab 12: 2042018820980225.
- Protic DD, Aishworiya R, Salcedo-Arellano MJ, Tang SJ, Milisavljevic J, et al. (2022) Fragile X Syndrome: From Molecular Aspect to Clinical Treatment. Int J Mol Sci 23(4): 1935.
- Lightbody AA, Bartholomay KL, Jordan TL, Lee CH, Miller JG, et al. (2022) Anxiety, Depression, and Social Skills in Girls with Fragile X Syndrome: Understanding the Cycle to Improve Outcomes. J Dev Behav Pediatr 43(9): e565-e572.
- Tabolacci E, Pomponi MG, Remondini L, Pietrobono R, Orteschi D, et al. (2021) Co-Occurrence of Fragile X Syndrome with a Second Genetic Condition: Three Independent Cases of Double Diagnosis. Genes (Basel) 12(12): 1909.
- Dölen G, Bear MF (2008) Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586(6): 1503-1508.
- Ligsay A, Van Dijck A, Nguyen DV, Lozano R, Chen Y, et al. (2017) A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome. J Neurodev Disord 9(1): 26.
- Utari A, Chonchaiya W, Rivera SM, Schneider A, Hagerman RJ, et al. (2010) Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil 115(5): 433-443.
- Greiss Hess L, Fitzpatrick SE, Nguyen DV, Chen YC, Gaul KNG, et al. (2016) A Randomized, Double-Blind, Placebo-Controlled Trial of Low-Dose Sertraline in Young Children With Fragile X Syndrome. J Dev Behav Pediatr 37(8): 619-628.
- Ligsay A, Hagerman RJ (2016) Review of targeted treatments in fragile X syndrome. Intractable Rare Dis Res 5(3): 158-167.
- Monyak RE, Emerson D, Schoenfeld BP, Zheng X, Chambers DB, et al. (2017) Insulin signaling misregulation underlies circadian and cognitive deficits in a Drosophila fragile X model. Mol Psychiatry 22(8): 1140-1148.
- Wang Y, Zhao J, Guo FL, Gao X, Xie X, et al. (2020) Metformin Ameliorates Synaptic Defects in a Mouse Model of AD by Inhibiting Cdk5 Activi-ty. Front Cell Neurosci 14: 170.
- Gantois I, Popic J, Khoutorsky A, Sonenberg N (2019) Metformin for Treatment of Fragile X Syndrome and Other Neurological Disorders. Annu Rev Med 70: 167-181.
- Dy ABC, Tassone F, Eldeeb M, Salcedo-Arellano MJ, Tartaglia N, et al. (2018) Metformin as targeted treatment in fragile X syndrome. Clin Genet 93(2): 216-222.
- Biag HMB, Potter LA, Wilkins V, Afzal S, Rosvall A, et al. (2019) Metformin treatment in young children with fragile X syndrome. Mol Genet Genomic Med 7(11): e956.
- Protic D, Aydin EY, Tassone F, Tan MM, Hagerman RJ, et al. (2019) Cognitive and behavioral improvement in adults with fragile X syndrome treated with metformin-two cases. Mol Genet Genomic Med 7(7): e00745.
- Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, et al. (2012) Psychometric study of the Aberrant Behavior Checklist in Fragile X Syndrome and implications for targeted treatment. J Autism Dev Disord 42(7): 1377-1392.
- American Diabetes Association (2022) Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes 1 January 40(1): 10-38.
- Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ (2002) Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 25(1): 89-94.
- Seifarth C, Schehler B, Schneider HJ (2013) Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes 121(1): 27-31.
- Soukas AA, Hao H, Wu L (2019) Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol Metab 30(10): 745-755.
- Esfahanian N, Shakiba Y, Nikbin B, Soraya H, Maleki-Dizaji N, et al. (2012) Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol Med Rep 5(4): 1068-1074.
- Salcedo-Arellano MJ, Dufour B, McLennan Y, Martinez-Cerdeno V, Hagerman R (2020) Fragile X syndrome and associated dis-orders: Clinical aspects and pathology. Neurobiol Dis 136: 104740.
-
Van Kim Ma*, Ramkumar Aishworiya, Elysa J Marco and Randi Hagerman. Effects of Metformin on Individuals with Fragile X Syndrome and Additional Genetic Variants. Arch Clin Case Stud. 4(3): 2024. ACCS.MS.ID.000588.
-
Genetic variants; fragile X syndrome; autism spectrum disorder; strabismus; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






