 Opinion
Opinion
Childhood Traumatic Events Accelerates Biological Aging
Hongyu Ni1 and Xiaohui Wu1,2*
1Department of Medical Genetics, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China
2Experimental Education/Administration Center, School of Basic Medical Science, Southern Medical University, Guangzhou, China
Xiaohui Wu, Department of Medical Genetics, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China
Received Date:May 05, 2024; Published Date:May 09, 2024
Opinion
Childhood traumatic events are a global public health issue, perhaps with potential lifelong physical health problems for those who experience them [1,2]. A report published by the Center on the Developing Child at Harvard University shows that the more childhood trauma a person accumulates, the more likely he is to develop a wide range of psycho-spiritual and cardiovascular disorders later in life [3]. However, it is less well understood what processes may be affected by adverse early experiences that sustain long-term health risks. Epigenetics may offer some explanation. Epigenetic processes allow the body to respond to environmental influences by regulate gene transcription by chemical modifications of either DNA or the structural components of chromatin without inducing changes to the DNA sequence [4]. DNA methylation is one of the most commonly studied epigenetic processes. Recently, mounting studies have confirmed that the strong correlation between childhood trauma and DNA methylation. Methylated gene traces in the blood of traumatised children significantly predicted the approximate incidence of health problems such as depression, tobacco addiction, alcoholism, etc., approximately 17 years later, with the help of Methylation Risk Scores (MRSs) [5].
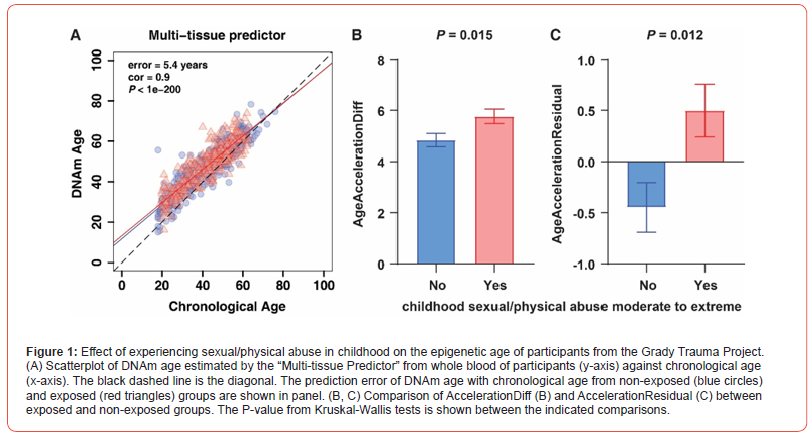
Methylation of the glucocorticoid receptor (GR) gene, NR3C1, is associated with behavioural problems and symptoms in children exposed to early adversity [6], as well as maltreatment as a predictor of changes in NR3C1 methylation over time [7]. However, these studies have not converted altered DNA methylation due to childhood trauma into quantitative numerical values of their effects on the development and final outcome of individual. The epigenetic clock is a molecular estimator developed based on DNA methylation data. It measures the cumulative effects of epigenetic maintenance systems, reflecting the dynamic landscape of interactions between genetic and environmental factors [8]. It can accurately estimate the biological age of any tissue to measure physiological homeostasis and degradation throughout the life course, which enables quantitatively assess the impact of longterm environmental stresses on individual homeostasis [9]. We quantified the epigenetic age (DNAm age) of whole blood from participants in the Grady Trauma Project [10], using the most widely used epigenetic clock “Multi-Tissue Predictor”, which is a predictor of 353 CpG sites generated by applying an elastic net regression model based on Illumina DNAm array data from thousands of samples (n > 8000) from 51 different tissues and cell types.
The results showed that the error value of DNAm age compared to chronological age was 5.4 years with a correlation coefficient of 0.9 (P < 1e-200, Figure 1A). We calculated epigenetic age acceleration by calculating Acceleration Diff and Acceleration Residual and determined whether participants’ epigenetic age was older (epigenetic age acceleration > 0) or younger (epigenetic age acceleration < 0) than their chronological age. Next, we used the Kruskal-Wallis’s test to determine whether experiencing moderate to extreme sexual/physical abuse in childhood would have a significant effect on an individual’s epigenetic age acceleration. We observed that the Acceleration Diff value (increased by 0.93 years; p = 0.015, Figure 1B) and Acceleration Residual (p = 0.012, Figure 1C) values displayed significant rise in individuals who experienced abuse in childhood compared to those who did not. This result suggests that exposure to adverse childhood experiences may lead to an earlier onset of signs of aging in later life through cumulative effects. Our study elucidates the relationship between childhood trauma and phenotypic aging measure. While everyone gets older, childhood adversity can cause individuals to age faster, which puts you at greater risk for physical health problems in adulthood.
\Therefore, studying a link between the methylation changes of the epigenetic clock and childhood trauma is even more clinically relevant, as these changes could potentially serve as biomarkers to identify children at risk for long term health problems. Our study elucidates the relationship between childhood trauma and phenotypic aging measure. While everyone gets older, childhood adversity can cause individuals to age faster, which puts you at greater risk for physical health problems in adulthood. Therefore, studying a link between the methylation changes of the epigenetic clock and childhood trauma is even more clinically relevant, as these changes could potentially serve as biomarkers to identify children at risk for long term health problems. Psychologist Alfred Adler said, Unfortunate people are healing their childhood all their lives, and lucky people are healed by childhood all their lives. The epigenetic clock provides a complete picture reflecting health inequalities between individuals caused by early life exposures [11]. Some studies even hint that epigenetic changes could affect the next generation [12]. A serious effort to both map and substantiate associations between adverse behavioural responses caused by childhood trauma and epigenetic clock alterations may provide possible treatments for reversing physical health problems triggered by childhood trauma.
Funding
We thank the National Natural Science Foundation of China [grant number 32000419] and the Guangdong Science and Technology Foundation [grant number 2023A1515012374] and Guangdong-Hong Kong Joint Laboratory for Psychiatric Disorders (2023B1212120004) for providing financial supports.
Acknowledgement
We gratefully acknowledge the many researchers who made their DNA methylation datasets publicly available and responded to my email requests. This study would not have been possible without the valuable data from the NCBI Gene Expression Omnibus database.
Conflict of Interest
The authors declare no conflict of interest.
References
- Ashwini Tiwari, Andrea Gonzalez (2018) Biological alterations affecting risk of adult psychopathology following childhood trauma: A review of sex differences. Clin Psychol Rev 66: 69-79.
- Nena Messina, Christine Grella (2006) Childhood trauma and women's health outcomes in a California prison population. Am J Public Health 96(10): 1842-1848.
- National Forum on Early Childhood Policy & Programs (2010) The Foundations of Lifelong Health Are Built in Early Childhood. Summary of Essential Findings.
- JK Kim, M Samaranayake, S Pradhan (2009) Epigenetic mechanisms in mammals. Cell Mol Life Sci 66(4): 596-612.
- Charlie LJD van den Oord, William E Copeland, Edwin JCG van den Oord, Min Zhao, Lin Ying Xie, et al. (2022) DNA methylation signatures of childhood trauma predict psychiatric disorders and other adverse outcomes 17 years after exposure. Mol Psychiatry 27(8): 3367-3373.
- Stephanie H Parade, Kathryn K Ridout, Ronald Seifer, David A Armstrong, Carmen J Marsit, et al. (2016) Methylation of the Glucocorticoid Receptor Gene Promoter in Preschoolers: Links with Internalizing Behavior Problems. Child Dev 87(1): 86-97.
- Justin Parent, Stephanie H Parade, Laura E Laumann, Kathryn K Ridout, Bao-Zhu Yang, et al. (2017) Dynamic stress-related epigenetic regulation of the glucocorticoid receptor gene promoter during early development: The role of child maltreatment. Dev psychopathol 29(5): 1635-1648.
- Steve Horvath (2013) DNA methylation age of human tissues and cell types. Genome Biol 14(10): R115.
- Elaine E Guevara, Richard R Lawler (2018) Epigenetic Clocks. Evol Anthropol 27(6): 256-260.
- Xingqi Cao, Chao Ma, Zhoutao Zheng, Liu He, Meng Hao, et al (2022) Contribution of life course circumstances to the acceleration of phenotypic and functional aging: A retrospective study. EClinicalMedicine 51: 101548.
- Eric J Nestler (2012) Stress makes its molecular mark. Nature 490(7419): 171-172.
-
Hongyu Ni and Xiaohui Wu*. Childhood Traumatic Events Accelerates Biological Aging. Arch Clin Case Stud. 4(1): 2024. ACCS.MS.ID.000578.
-
Biological aging; psycho-spiritual; DNA sequence; DNA methylation; multi-tissue predictor; childhood trauma; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






