 Research Article
Research Article
The Use of Spinal Cord Stimulators for the Treatment of Abdominal Pain: A Comprehensive Review
Omar Alnatour1*, Saba Javed2
1University of Texas Health Science Center at Houston, Texas, USA
2Department of Pain Medicine, University of Texas, MD Anderson Cancer Center, Houston, Texas, USA
Omar Alnatour, University of Texas Health Science Center at Houston, Texas, USA
Received Date:January 12, 2024; Published Date:January 26, 2024
Abstract
Introduction: Abdominal pain is a challenging condition to treat due to its broad etiology, often refractory response to medical management,
and often negative workup. Spinal cord stimulators (SCS) are a known gold standard for refractory chronic pain conditions, but their published use
in the treatment of abdominal pain is relatively rare. Therefore, a literature search was undertaken from January 2000 through December 2022
and twenty-seven relevant articles were included in this review. Our goal was to provide a comprehensive review and source of information for
interventional pain physicians on available literature regarding abdominal pain and the use of SCS for its treatment.
Objectives: The purpose of this article is to review the current use of spinal cord stimulators in the setting of abdominal pain and support its
continued use.
Study design: This is a narrative review article with the goal of reviewing pertinent case reports, case series, prospective and retrospective
studies, from January 2000 to December 2022 on the use of spinal cord stimulators in the treatment of abdominal pain.
Conclusion: Overall, the use of SCS resulted in decreased abdominal pain scores, relief of gastrointestinal symptoms, decreased morphine
milligram equivalent requirements, and improved quality of life. These results support the initiation of randomized controlled trials in order to
establish strong evidence for their use in the treatment of abdominal pain.
Keywords:Abdominal pain; spinal cord stimulator; neuromodulation; pain management; chronic pain
Key Points:a) Over two decades of articles were included in this review on the use of spinal cord stimulators for the treatment of abdominal pain.
b) The use of spinal cord stimulator was found to decrease abdominal pain scores in the patient population reviewed.
c) The use of spinal cord stimulator was found to decrease morphine milligram equivalent requirements in the patient population reviewed.
d) The use of spinal cord stimulator was also found to decrease gastrointestinal symptoms, such as nausea, vomiting, diarrhea, and constipation
in the patient population reviewed.
e) The use of spinal cord stimulator was found to improve quality of life and daily functional ability in the patient population reviewed.
f) Given the lack of randomized control trials on the use of spinal cord stimulators for abdominal pain, the evidence for its use is considered
insufficient, although can result in improvement.
Background
Abdominal pain is a challenging condition for primary care providers, gastrointestinal specialists, and chronic pain providers to treat due to the broad-ranging differentials of its etiology and often refractory response to medical management in combination with often negative workup. Additionally, patients often have debilitating gastrointestinal symptoms that in combination with their refractory pain, have profound negative effects on psychological well-being and quality of life. The etiologies of abdominal pain vary and can be divided into the four subtypes, which are functional gastrointestinal disorders, abdominal pain of a visceral origin, abdominal wall pain conditions, and abdominal pain syndromes that result from generalized diseases. Additionally, abdominal pain can also be viewed from a viewpoint of being either neuropathic or nociceptive in origin. In neuropathic pain, according to the International Association for the Study of Pain (IASP), there is a lesion or disease of the somatosensory nervous system that results in pain.
Nociceptive pain, in contrast, arises from actual or threatened damage to non-neural tissues and is due to the activation of nociceptors. The pathophysiology of abdominal pain is incredibly complex and includes many overlapping components such as the “Brain-gut axis”, the dorsal column, unmyelinated C fibers, and alpha-delta pain fibers. The “Brain-gut axis” describes the bidirectional neural circuit which integrates peripheral input (sensory, motor, and autonomic) and central nervous system input to end organs in addition to its role in gastrointestinal physiology [1]. It had previously been believed that abdominal pain was nociceptive in origin however, recent evidence points to a neuropathic nature. Additionally, the dorsal column plays a key role in both the transmission and modulation of visceral pain and can be subject to changes in signal transduction from conditions that result in chronic stimulation or inflammation of peripheral nerves. A more detailed discussion on this topic can be found in the abovementioned textbook by Dr. Kapural [2].
Current treatments for chronic abdominal pain are wideranging and include lifestyle changes, pain-targeted psychotherapy, cognitive behavioral therapy, non-opioid analgesics, neuropathic medications, non-steroidal anti-inflammatory drugs, muscle relaxants, opioid medications, epidural steroid injection, nerve blocks, medial branch blocks, radiofrequency ablations, peripheral stimulation, and even surgery. Spinal cord stimulators have long been commonly used as the gold standard to treat a variety of refractory chronic pain conditions, however their use in the treatment of abdominal pain is still currently relatively rare and even less studied or published. Herein, we present the most comprehensive review, including case reports, case series, retrospective studies, prospective studies, national surveys, randomized crossover studies, and trials published in English from January 2000 to December 2022 on the use and effectiveness of spinal cord stimulators for the treatment of abdominal pain and presented our findings.
Materials and Methods
Criteria For Considering Studies for This Review
a) Types of studies: Case reports, case series, retrospective
studies, prospective studies, national surveys, and randomized
crossover studies, published in English from January 2000 to
December 2022 were included, to evaluate the most current
literature.
b) Types of participants: The patients included in this review
varied in age, gender, ethnicity, etiology of abdominal pain, and
characterization of abdominal pain.
c) Types of interventions: This review exclusively focused on
the use of spinal cord stimulators as the intervention.
d) Types of outcomes: Given the non-standardization of
outcome measures across the articles included in this review,
specific outcome measures were not an inclusion criteria. This
review mainly focused on abdominal pain scores, morphine
milligram equivalent (MME) requirements, gastrointestinal
symptom relief, and quality of life as primary outcome
parameters.
Methods
We performed a comprehensive search using systematic review search methods adhering to the PRISMA-S for Searching checklist [3]. We searched Ovid MEDLINE, Ovid EMBASE, Cochrane Central, and Web of Science. Databases were searched from inception to December 29, 2022. Search structures, subject headings, and keywords were tailored to each database by a medical research librarian (KJK) specializing in systematic reviews in consultation with co-authors. Searches were restricted to English language articles but were not restricted by any other type of limit. We included grey literature resources such as conferences, dissertations, reports, and other unpublished studies for additional relevant citations. Deduplication was performed manually in Endnote. The full search strings for all databases can be found in supplementary (Table 1).
Selection Process
After the initial search, Rayyan software (Rayyan Systems Inc, Cambridge, Massachusetts) was used to screen the citations. Two of the principal investigators (OA, SJ) independently screened the titles and abstracts of the articles to identify potentially relevant studies. Disagreements were resolved by consensus. Studies that passed the title/abstract review were retrieved for full-text review. The two investigators (OA, SJ) then independently screened the remaining full-text articles. Disagreements were resolved by consensus.
Eligibility Criteria
We included case reports, case series. retrospective studies, prospective studies, and national surveys reporting on adult patients (age 18 and above) with any form of non-acute abdominal pain of any origin that underwent treatment with SCS. Primary outcomes were focused on pain scores and gastrointestinal symptom relief. Secondary outcomes included impacts on quality of life, disability, activities of daily living, and pain interference. Review articles, meta-analyses studies, and conference abstracts were not included. We also excluded animal studies, studies performed before 2000, studies that were not in English, studies reporting on pelvic pain, and studies that involved dorsal root ganglion stimulators as the intervention utilized. We identified and linked multiple reports of the same study, and we excluded them if they were duplicated or not relevant. We combined reports that described different findings from the same study and excluded papers that reported results that had already been published.
Data Collection
We extracted data on the following: type of study, year published, language published, number of patients in each study, abdominal pain type and origin, stimulator lead type and lead tip position, type of waveform utilized, and primary and secondary outcomes after SCS placement. Two review authors (OA, SJ) independently extracted data using a data extraction form created with Excel. We resolved any disagreements by discussion. We considered studies to have sufficient data if at least one data point was discussed in the data categories mentioned above. Studies were presented in a table by year with information on the aforementioned data presented in an organized fashion. In our discussion, we grouped studies by types of abdominal pain: mesenteric ischemia, irritable bowel syndrome, chronic pancreatitis, etc.
Results
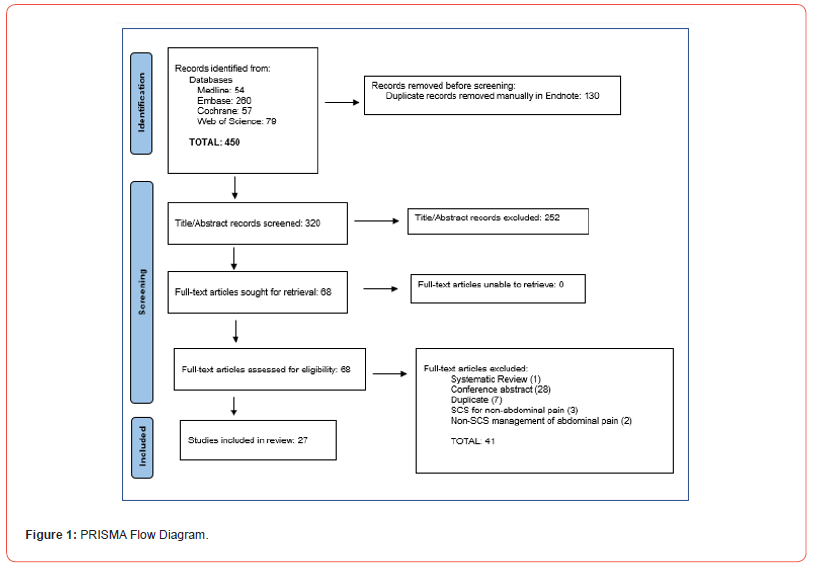
We retrieved 450 unique articles for review. Of these, 27 studies met all the criteria for inclusion in this systematic review. These included 14 case reports, 5 case series, 5 retrospective studies, 1 national survey, 1 randomized crossover pilot study, and 1 prospective study. The PRISMA flow diagram (Figure 1) shows the entire selection process from the original search results to the final selection of studies (Table1).
Table 1:Search Strings.

Results
Search Results
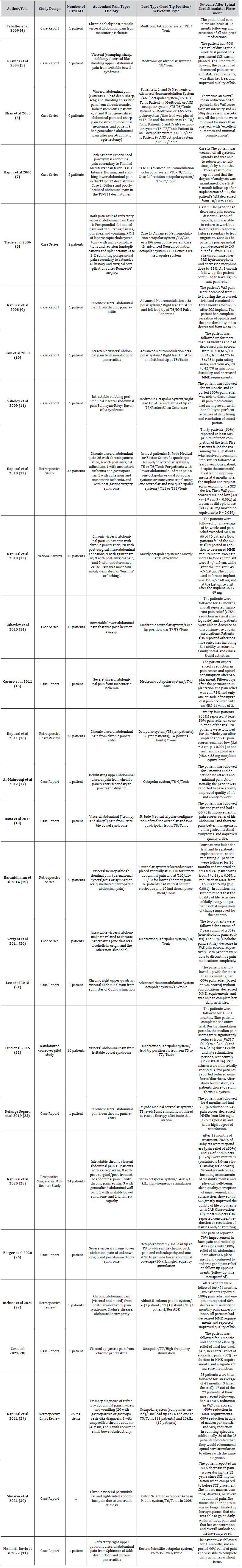
A total of 450 relevant articles were identified in the literature search (Figure 1). Of these articles, 27 were found to meet the inclusion criteria of the focus of this review and were included. Articles that were excluded were those that did not include abdominal pain as the primary patient presentation, those that did not include the use of a spinal cord stimulator in the treatment of abdominal pain, studies on non-human subjects, review papers, and those that were published in any language other than English. The included studies are the following: 14 case reports, 5 case series, 5 retrospective studies, 1 national survey, 1 randomized crossover pilot study, and 1 prospective study (Table 2).
Table 2:Summary of Articles on the Use of Spinal Cord Stimulators for the Treatment of Abdominal Pain.
Discussion
Our literature search was focused on the effect of spinal cord stimulators in the treatment of any type of abdominal pain and resulted in 27 unique articles over the past two decades. The causes of abdominal pain were diverse, the characterization of the pain varied but was mostly visceral, and the location of pain varied as it was poorly localized. The most common lead type used was an octopolar lead system and the most common lead tip position was the T6 to T8 region. Figure 2 below shows the abdominal dermatomes in relation to the abdominal organs and Figures 3 and 4 below show a graphic of general abdominal dermatome coverage in relation to SCS lead tip placement and location based on the studies included in this article. All patients included received a SCS trial and were only given a permanent SCS if they had a significant reduction in abdominal pain scores during the trial. The follow-up time after permanent SCS implantation varied from one month to seven years but was approximately 12 months in most articles. For the majority of articles, VAS scores were used in pain assessment and the results varied from >50% relief to complete analgesia.
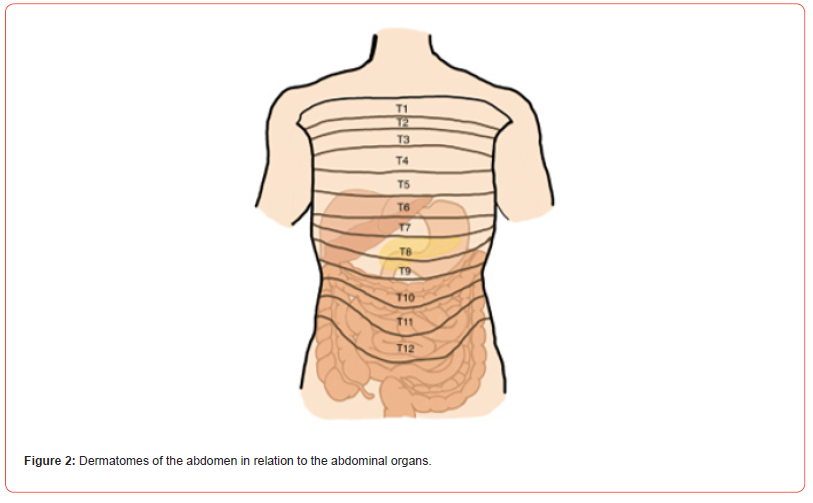
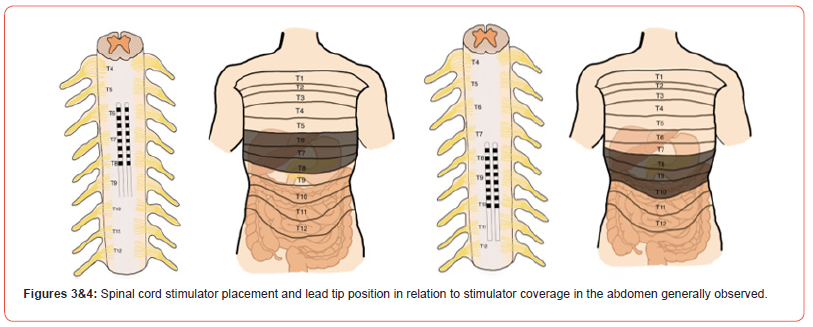
In every article review, there is mention of decreased morphine milligram equivalent (MME) requirements. Additionally, many of the articles mention gastrointestinal symptom relief, improvement in quality of life, and ability to return to daily activities (Figures 2-4). The below discussion has the 27 included literature findings grouped by specific etiology of abdominal pain to provide a useful perspective in assessing the efficacy of SCS in treating abdominal pain. This includes mesenteric ischemia, postprandial abdominal pain, irritable bowel syndrome, chronic pancreatitis, more rare etiologies such a familial Mediterranean fever, Bannayan-Riley- Ruvalcaba Syndrome, and sphincter of Oddi dysfunction, and larger studies that focused on visceral abdominal pain of various etiologies. Pain due to mesenteric ischemia can be debilitating in nature and challenging to manage. Ceballos et al and Caruso et al both published case reports of patients with severe abdominal pain due to mesenteric ischemia that was refractory to other medical management who underwent SCS and found complete analgesia and cessation of all other analgesics [4-15].
The discussed mechanisms were thought to be due to inhibition of nociceptive input, decreased secondary sympathetic activity, release of prostaglandins and endorphins that play a role in modulation of nociceptive transmission, and induction of peripheral perfusion in occlusive and vasospastic peripheral arteriopathies. In 2006, Tiede et al presented a patient with refractory postprandial abdominal pain although not from mesenteric ischemia in origin, that reported >50% reduction in pain scores and decreased MME requirements (discontinuation of breakthrough hydromorphone and 33% decrease in morphine dose) at three-month follow-up [16]. Irritable bowel syndrome is an equally challenging condition to manage. In 2004, Krames et al presented a 50-year-old female with a 30-year history of abdominal pain and diarrhea secondary to IBS that experienced decreased pain scores, decreased MME requirements, cessation of diarrhea, and improved quality of life during a ten-month follow-up after SCS placement [17].
The authors discussed the visceral hypersensitivity to luminal distention that occurs in IBS, the role of spinothalamic tracts in the context of chronic pain that is of visceral origin, the role that postsynaptic dorsal column pathways may play in pain signal amplification, and the role of SCS to increase blood flow via antidromic activation of sensory afferents and resulting neuromodulators substance release. Similarly, in 2012, Rana et al presented a case report on a 36-year-old male with an eightyear history of “crampy and sharp” abdominal pain secondary to constipation predominant IBS which had failed conservative therapy including opioids and psychologic treatment [18]. The patient received a SCS with the lead tip at T8 and was followed for one year during which he reported greater than 60-70% improvement in pain scores, improvement in IBS symptoms, improved quality of life, and ability to function at work. Moreover, in a randomized crossover pilot study of ten patients with chronic abdominal pain secondary to IBS who received a SCS with lead tip at T5-T8 and were followed over 28 weeks, nine completed the trial and reported decreased VAS pain scores (from 7 to 3 and to 4 during early and late stimulation periods, respectively, P <0.03- 0.04), decreased pain attacks, and reduced episodes in diarrhea in some patients [19-22].
Chronic pancreatitis is another debilitating diagnosis and can be extremely difficult to manage the pain. In 2005, Khan et al present nine patients with varying etiologies of abdominal pain including chronic pancreatitis, generalized abdominal pain, abdominal wall neuroma, and post-traumatic splenectomy. All the patients received a SCS and were followed for more than one year in which all with an overall mean reduction of 4.9 points in the VAS score for pain and >50% in MME requirements. The authors discuss the importance of dermatomal paresthesia, in the context of abdominal visceral pain modulation, in order to ensure concordance with the viscerotomal nervous distribution of the various abdominal pain conditions in their patients. This was reflected by pancreatic pain covered via SCS placement at T5-T6 and post-splenectomy pain coverage via SCS placement at T6-T7. Moreover, Kapural et al. published a retrospective chart review of 30 patients (20 females and 10 men) with chronic pancreatitis (for an average of 7.8 years +/- 5 years) that was epigastric in location for most and sharp, stabbing, and aching in nature.
24 patients reported at least 50% pain relief with the trial (6 failed the trial) and, of them, 20 patients received an implant (one patient was lost to follow-up and three were removed due to infection). The 20 patients were followed for a year and continued to report both greater than 50% reduction in pain scores in addition to decreased MME requirements [23]. More recently, In 2020, Kapural et al presented the first prospective (12- month, single-arm) study on the safety and efficacy of SCS in 24 patients with intractable chronic abdominal pain [24]. The diagnosis of the patients varied (see Table 1 above), average age of 47.7, gender majority female (19 out of 24), and average diagnosis duration was 7.8 years. With the SCS trial (leads placed from T4-T8), 23 out of the 24 patients reported at least 70% pain relief and these 23 patients received an SCS implant. After a 12-month follow-up, 78.3% of the patients reported 50% or more pain relief as well as improvements in many patients in secondary outcomes of functional capacity, quality of life, sleep, and gastrointestinal symptom relief [25]. In addition to the widely accepted gate control theory of pain as a mechanism through which SCS provides benefit in this setting, other mechanisms presented include neural conduction blockade, activation of putative supraspinal pain centers, supraspinal or intraspinal sympathetic blockade, and release of neuromodulators.
Of note, Ranayake et al published a thorough systematic review in 2019 focused on the utilization of SCS in treating abdominal pain specifically from chronic pancreatitis. The seven articles from that systematic review are included in this article. SCS has shown beneficial for rare causes of abdominal pain as well. In 2006, Kapur et al presented two patients with paroxysmal abdominal pain, fever, nausea, and vomiting secondary to familial Mediterranean fever. Both patients received a SCS, and both reported decreased VAS pain scores and decreased MME requirements. In 2009, Yakovlev et al presented an eighteen-year-old female with intractable chronic periumbilical abdominal pain secondary to Bannayan-Riley- Ruvalcaba Syndrome that had failed conservative therapy, extensive workup, and medical management. The patient received a SCS at T6-T7 and reported >50% decrease in pain scores, discontinued all pain medications, had an improvement in her ability to perform daily activities, and resolution of constipation.
The following year, Yakovlev et al presented a case series of 15 patients with intractable abdominal pain that was postherniorrhaphy. The patients received a SCS at T7-T9 and were followed for 12 months after which they all reported significant pain relief (>75% reduction in visual analog scale) and all patients were able to either decrease or discontinue use of pain medications. In 2015, Lee et al presented a case report of a 58-year-old female with chronic right upper quadrant abdominal pain due to sphincter of Oddi dysfunction that received a SCS at T5-7. She was followed for more than six months and reported >50% reduction in VAS pain scores and decreased MME requirements. Mamaril-Davis et al presented a case report, most recently, in 2022 on a patient in their thirties with chronic abdominal pain secondary to medically refractory sphincter of Oddi dysfunction and chronic pancreatitis.
The had as successful seven-day SCS trial and underwent permanent SCS placement with dual octopolar electrodes at T6-7 and reported, at 18-month follow-up, 90% pain relief and ability to return to normal daily activities. In 2020, Berger et al presented a case report on a 56-year-old male with chronic severe lower abdominal pain and chronic back pain that had failed medical management, psychotherapy, cognitive behavior therapy, lumbar and epidural steroid injections, and nerve blocks [26]. The patient received two 8-contact leads placed (one at T8 to address the chronic back pain and one at T6 for lower abdominal pain coverage). The patient reported 100% relief of abdominal pain and 70% relief of back pain during follow-up appointments. That same year, Richter et al presented a clinical series of 3 patients with chronic abdominal pain of different origins (post-herniorrhaphy pain syndrome, Crohn’s disease, and intercostal neuralgia) that received a 5-column paddle SCS (one patient at T6, one patient at T7, and one patient at T8 for lead tip position) [27].
The patients were followed for at least two years and two patients reported being entirely pain free. The third patient reported a 60% decrease in pain severity, a 33% in frequency of the monthly pain exacerbations, and was able to completely discontinue all opioids. All three patients reported an improvement in quality of life. In 2021, Shearin et al presented a case report on a 57 year-old female with over 20 years of visceral abdominal pain of uncertain etiology that was refractory to medical management and multiple interventional pain procedures [28]. The patient received a paddle SCS at T6/T7 and during 12 years of follow-up, reported a 80% decrease in pain scores since SCS implantation when compared to before SCS placement and improved quality of life. The authors thus propose that SCS, in the context of the aforementioned mechanisms it is believed to decrease visceral pain, as an alternative treatment in patients with chronic abdominal pain that is refractory to nonsurgical interventions.
In 2021, Kapural et al presented a retrospective chart review of 26 patients that underwent a SCS trial for a primary diagnosis of refractory abdominal pain, nausea, and vomiting. 23 of the 26 patients reported >50% reduction in pain after the trial and were followed for an average of 41 months after permanent SCS implantation (either low or high frequency devices used) [29]. At last follow-up, there was a >50% reduction in pain scores and MME requirements. Additionally, the days of nausea per month decreased from 26.3 days to 11.7 days per month. 20 out of the 23 patients reported being satisfied with their therapy and would recommend it to others with the same diagnosis. The authors note that the improvement in nausea and vomiting seen in their patients may be related to decreased opioid requirements, the antiemetic effects from gastric physiology modulation, and possibly improvement in gastric emptying among the majority of patients that had gastroparesis.
There were, however, select cases where the use of SCS did not provide relief of abdominal pain. In 2006, Tiede et al presented a patient with chronic abdominal pain that ultimately had response failure to SCS due to lead migration from a fall. Kapural et al reported five patients in 2010 that failed SCS trial that were later trialed on alternative therapies. In a 2011 retrospective chart review, Kapural et al reported six patients that failed SCS trial, with mention of those patients having a higher rate of depression, alcoholism, and poor response to sympathetic nerve block. In 2014, Baranidharan et al reported four patients that failed SCS trial with mention that initial failure of neuromodulation was seen in patients that had a lack of response to sympathetic blocks and exhibited opioid-seeking behaviors [30,31]. This review, although is the most comprehensive of its kind to date, does have some limitations.
This selected timeframe was purposely chosen in order to review the early use of SCS for abdominal pain and track its progression in the context of patient outcomes to present date. This review article included literature published only in English. For the relevant articles that were included in this narrative review, the sample size was relatively small due to the still relatively rare use of spinal cord stimulators for the treatment of abdominal pain. The characterization and location of abdominal pain varied amongst the review patient population and it is recognized that, in discussing outcomes, pain is subjective. Additionally, the articles presented may not be fully representative of the experience with SCS in the treatment of abdominal pain due to negative publication bias. Lastly, the time to follow-up between patients varied and is included in Table 1 above.
Conclusion
In our review we present the most comprehensive review of SCS on abdominal pain to date. Although the patients were heterogenous in age along with gender and the etiologies of abdominal pain in the patients reviewed varied, along with the location and type of pain experienced, the majority of patients in this review experienced a benefit from SCS placement. This was supported by decreased pain scores, decreased morphine milligram equivalent requirements, reported relief of gastrointestinal symptoms, improvement in quality of life, and improvements in daily functional ability. As the use of SCS for the treatment of chronic abdominal pain is currently considered “off-label” by The United States Food and Drug Association, this review supports the commencement of randomized controlled trials to further explore SCS as a treatment option for chronic abdominal pain.
Financial & Competing Interests Disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Acknowledgement
None.
Conflict of interest
No conflict of Interest.
References
- Sabo C M, Grad S, Dumitrascu DL (2021) Chronic Abdominal Pain in General Practice. Dig Dis 39(6): 606-614.
- Leonardo, Kapural (2015) Chronic Abdominal Pain: An Evidence-Based, Comprehensive Guide to Clinical Management. Springer, New York, USA. pp. 1379-1385.
- Rethlefsen M L, Kirtley S, Waffenschmidt S (2021) PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev 10(1): 39.
- Ceballos A, Cabezudo L, Bovaira M, Fenollosa P, Moro B (2000) Spinal cord stimulation: a possible therapeutic alternative for chronic mesenteric ischaemia. Pain 87(1): 99-101.
- Krames E, Mousad D G (2004) Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: a case report. Neuromodulation 7(2): 82-88.
- Khan YN, Raza SS, Khan EA (2005) Application of spinal cord stimulation for the treatment of abdominal visceral pain syndromes: case reports. Neuromodulation 8(1): 14-27.
- Kapur S, Mutagi H, Raphael J (2006) Spinal cord stimulation for relief of abdominal pain in two patients with familial Mediterranean fever. Br J Anaesth 97(6): 866-868.
- Tiede J M, Ghazi S M, Lamer T J, Obray J B (2006) The use of spinal cord stimulation in refractory abdominal visceral pain: case reports and literature review. Pain Pract 6(3): 197-202.
- Kapural L, Rakic M (2008) Spinal cord stimulation for chronic visceral pain secondary to chronic non-alcoholic pancreatitis. J Clin Gastroenterol 42(6): 750-751.
- Kim J K, Hong S H, Kim M H, Lee J K (2009) Spinal Cord Stimulation for Intractable Visceral Pain due to Chronic Pancreatitis. J Korean Neurosurg Soc 46(2): 165-167.
- Yakovlev A E, Resch B E (2009) Treatment of intractable abdominal pain patient with Bannayan-Riley-Ruvalcaba syndrome using spinal cord stimulation. WMJ 108(6): 323-326.
- Kapural L, Nagem H, Tlucek H, Sessler DI (2010) Spinal cord stimulation for chronic visceral abdominal pain. Pain Med 11(3): 347-355.
- Kapural L, Deer T, Yakovlev A, Bensitel T, Hayek S, et al. (2010) Technical aspects of spinal cord stimulation for managing chronic visceral abdominal pain: the results from the national survey. Pain Med 11(5): 685-691.
- Yakovlev AE, Al Tamimi M, Barolat G, Karasev SA, Merkulov YA, et al. (2010) Spinal cord stimulation as alternative treatment for chronic post-herniorrhaphy pain. Neuromodulation 13(4): 288-290.
- Caruso C, Lo Sapio D, Ragosa V, Lo Sapio S, Cafora C, et al. (2011) Abdominal angina due to obstruction of mesenteric artery treated with spinal cord stimulation: a clinical case. Neuromodulation 14(2): 146-149.
- Kapural L, Cywinski JB, Sparks DA (2011) Spinal cord stimulation for visceral pain from chronic pancreatitis. Neuromodulation 14(5): 423-426.
- Al-Mahrouqi H, Munro Z, Acland RH, MacFarlane MR (2012) Spinal cord stimulation for intractable chronic upper abdominal pain: a case report of the first patient in New Zealand. N Z Med J 125(1367): 132-134.
- Rana MV, Knezevic NN (2013) Tripolar spinal cord stimulation for the treatment of abdominal pain associated with irritable bowel syndrome. Neuromodulation 16(1): 73-77.
- Baranidharan G, Simpson KH, Dhandapani K (2014) Spinal cord stimulation for visceral pain--a novel approach. Neuromodulation 17(8): 753-758.
- Vergani F, Boukas A, Mukerji N, Nanavati N, Nicholson C, et al. (2014) Spinal cord stimulation for visceral pain related to chronic pancreatitis: report of 2 cases. World Neurosurg 81(3-4): 651.
- Lee KH, Lee SE, Jung JW, Jeon SY (2015) Spinal cord stimulation for intractable visceral pain due to sphincter of oddi dysfunction. Korean J Pain 28(1): 57-60.
- Lind G, Winter J, Linderoth B, Hellström PM (2015) Therapeutic value of spinal cord stimulation in irritable bowel syndrome: a randomized crossover pilot study. Am J Physiol Regul Integr Comp Physiol 308(10): R887-894.
- Delange Segura L, Rodríguez Padilla M, Palomino Jiménez MT, Fernández Baena M, Rodríguez Staff JF (2019) Salvage Therapy with Burst Spinal Cord Stimulation for Chronic Pancreatitis: A Case Report. Pain Pract 19(5): 530-535.
- Ratnayake CB, Bunn A, Pandanaboyana S, Windsor JA (2020) Spinal Cord Stimulation for Management of Pain in Chronic Pancreatitis: A Systematic Review of Efficacy and Complications. Neuromodulation 23(1): 19-25.
- Kapural L, Gupta M, Paicius R, Strodtbeck W, Vorenkamp KE, et.al, (2020) Treatment of Chronic Abdominal Pain With 10-kHz Spinal Cord Stimulation: Safety and Efficacy Results From a 12-Month Prospective, Multicenter, Feasibility Study. Clin Transl Gastroenterol 11(2): e00133.
- Berger AA, Hasoon J, Urits I, Viswanath O, Gill J (2020) 10 kHz Spinal Cord Stimulation for Combined Alleviation of Post-Laminectomy Syndrome and Chronic Abdominal Pain: A Case Report. J Pain Res 13: 873-875.
- Richter B, Novik Y, Bergman JJ, Tomycz ND (2020) The Efficacy of BurstDR Spinal Cord Stimulation for Chronic Abdominal Pain: A Clinical Series. World Neurosurg 138: 77-82.
- Cox C J, Wilkinson MM, Erdek MA (2022) Successful spinal cord stimulation for chronic pancreatitis and post-laminectomy pain. Pain Manag 12(2): 123-129.
- Kapural L, Brown BK, Harandi S, Rejeski J, Koch K (2022) Effects of Spinal Cord Stimulation in Patients with Chronic Nausea, Vomiting, and Refractory Abdominal Pain. Dig Dis Sci 67(2): 598-605.
- Shearin E Alexander, Moufarrij Nazih A (2020) Twelve years of success in treating a patient with chronic visceral abdominal pain using paddle spinal cord stimulation. Interdisciplinary Neurosurgery 24(5): 101050.
- Mamaril-Davis JC, Aguilar-Salinas P, Balogun R, Weinand ME (2022) Spinal cord stimulation for medically refractory sphincter of Oddi dysfunction: A case report. Interdisciplinary Neurosurgery 28: 101487.
-
Omar Alnatour*, Saba Javed. The Use of Spinal Cord Stimulators for the Treatment of Abdominal Pain: A Comprehensive Review. Arch Clin Case Stud. 3(4): 2024. ACCS.MS.ID.000569.
-
Rheumatoid polyarthritis, Hypertensive, Diabetic, Anti-inflammatory drugs, Pancytopenia, Methotrexate.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






