 Case Report
Case Report
Rasburicase Induced Hemolysis in A Patient with Undiagnosed Glucose 6 Phosphate Dehydrogenase (G6PD) Deficiency
Lorie Ann Foster1*, Nancy Lin1, Ki Hong Oh2, Anna Andrzejczyk3 and Alison T Stopeck3
1Stony Brook Internal Medicine Residency Program, Stony Brook Medicine, USA
2Renaissance School of Medicine at Stony Brook University
2Division of Hematology and Oncology, Department of Medicine, Stony Brook Medicine
Lorie-Ann Foster, Stony Brook Internal Medicine Residency Program, Stony Brook Medicine, USA
Received Date:January 17, 2024; Published Date:February 01, 2024
Abstract
Rasburicase is a recombinant urate oxidase effective in lowering uric acid accumulation in patients with hematologic and solid tumor malignancies receiving anti-cancer therapy [1]. Its use has also been associated with increased financial burden and morbidity when used inappropriately. When used in patients who are deficient in glucose-6-phosphate dehydrogenase (G6PD), it often results in life threatening acute hemolytic anemia. We report an interesting case of a 74-year-old female treated with rasburicase for presumed tumor lysis syndrome (TLS) who later developed acute hemolytic anemia secondary to G6PD deficiency. Here we highlight and emphasize how to minimize inappropriate use of rasburicase and how to recognize patients with increased risk for developing G6PD deficiency
Background
Rasburicase is approved by the Food and Drug Administration (FDA) for management of hyperuricemia in patients with leukemia, lymphoma, and solid tumor malignancies who are receiving anticancer therapy at risk of developing tumor lysis syndrome (TLS). Rasburicase has several black box warnings associated with its use including severe hemolysis in patients with G6PD deficiency [2]. Patients deficient in G6PD are unable to breakdown hydrogen peroxidase, a byproduct of rasburicase, which causes oxidative stress to erythrocytes and triggers hemolysis [3]. The FDA recommends screening individuals at high risk for G6PD deficiency including those of African or Mediterranean ancestry prior to administration of rasburicase [4].
Case Report Summary
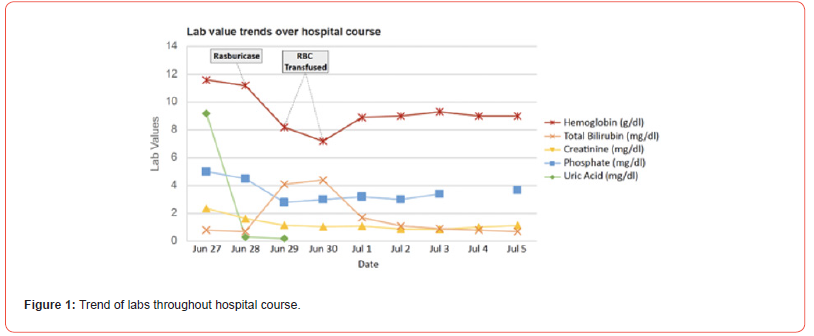
A 74-year-old female of Mediterranean decent with past medical history of metastatic pancreaticobiliary cancer with spread to the liver, lung and bones presented to the Emergency Department with hypovolemic shock and acute on chronic kidney injury. Other significant medical history included non-ischemic cardiomyopathy with reduced ejection fraction (35-40%) status post pacemaker placement, chronic kidney disease and anemia. Labs on admission (Figure 1) were notable for elevated uric acid (9.2 mg/dl), hyperphosphatemia (5 mg/dl) and elevated creatinine (2.37 mg/dl). She was given a dose of rasburicase for presumed TLS. Blood pressure was stabilized in the ICU (Intensive Care Unit) with fluids and vasopressors and the patient was subsequently transferred to the medical oncology service. Notably, due to worsening performance status, worsening kidney function, and overall resistance to treatment, the last dose of chemotherapy (gemcitabine) was administered 6 months prior to presentation.
The day following rasburicase treatment, the patient developed fatigue, jaundiced skin, scleral icterus, and tea-colored urine. Labs (Figure 1) showed a significant decrease in hemoglobin, elevated lactate dehydrogenase (LDH), hyperbilirubinemia, decreased haptoglobin and increased reticulocyte count consistent with hemolysis. A combos direct antibody test was negative. Because of progressive anemia, the patient required transfusion of three units of packed red blood cells before stabilizing. Notably, her G6PD levels were found to be low during this acute hemolytic episode, despite a high reticulocyte count. On medical history, in addition to Mediterranean descent, she disclosed multiple previous episodes of intermittent anemia requiring blood transfusion and several family members who were also diagnosed with unspecified anemia (Figure 1).
Discussion
G6PD deficiency affects upwards of 400 million people worldwide. Men are more commonly affected due to x-linked inheritance, but women can also be affected depending on lyonization. This results in two red blood cell populations based on the G6PD allele being expressed. The overall levels of G6PD enzyme activity in heterozygous females range from 30 to 80% of normal G6PD activity [5]. The commonly used qualitative tests do not discriminate intermediate G6PD activity which limits our understanding of the epidemiology of G6PD activity in females [6]. Furthermore, healthcare providers may perceive G6PD deficiency as having little or no impact on females which leads to missed opportunities to identify relatives with this genetic condition. Our patient was of Mediterranean descent and had a history of intermittent severe anemia which provided clues to consider her at risk of G6PD deficiency and hemolysis with the use of rasburicase. Rasburicase is used to treat and prevent hyperuricemia from TLS. TLS is an oncologic emergency characterized by metabolic derangements that include hyperkalemia, hyperphosphatemia, hyperuricemia, and hypocalcemia resulting in end organ damage [7].
The highest risk of TLS is seen in patients receiving chemotherapy for acute leukemia and lymphoma, whereas patients with solid malignancies carry a lower risk. Increased tumor burden defined by elevated white blood cells, elevated LDH, bulky disease, high proliferation rate, and organ metastases increase the risk of TLS [8]. Notably, in our case, the patient was not receiving anticancer therapy and had a tumor type unlikely to exhibit tumor lysis. Adverse drug events (ADEs) are the most common type of injury experienced by hospitalized individuals. It was found that errors were more likely to be intercepted if they occurred in the ordering stage rather than the administration stage [9]. Some commonly used medications to avoid in patients with G6PD deficiency include antimalarial drugs (chloroquine, primaquine), sulfamethoxazole and trimethoprim (bactrim), nitrofurantoin (macrobid), dapsone (aczone), colchicine and NSAIDs [10]. Other high-risk drugs associated with ADEs include anticoagulants, antihyperglycemic agents, sedatives, narcotics, and antipsychotics. These drugs share similar features including a narrow therapeutic window which requires close monitoring and dose adjustments to minimize errors [11].
It is therefore imperative to proceed with caution prior to administering these medications to reduce morbidity and prevent additional health care costs. Several groups have studied ways to maximize the cost-effectiveness of rasburicase [12]. These studies have noted provider errors such as lack of knowledge regarding TLS criteria, inconsistencies in ordering or reviewing labs, and proceeding without hematology-oncology consultation, to be associated with inappropriate or avoidable rasburicase use. In summary, this case illustrates the importance of employing a systemic approach to gathering patient information and history. The patient offered a personal and family history of anemia, was of Mediterranean descent, and had a clinical picture unlikely for TLS. By creating a broad differential diagnosis, the effect of anchoring bias is minimized. The patient’s electrolyte abnormalities could be explained by her worsening kidney function, rather than TLS, avoiding both the medical and financial toxicity of rasburicase.
Acknowledgment
None.
Conflict of interest
No conflict of Interest.
References
- Ueng S (2005) Rasburicase (Elitek): a novel agent for tumor lysis syndrome. Proc (Bayl Univ Med Cent) 18(3): 275-279.
- Cheah CY, Lew TE, Seymour JF, Burbury K (2013) Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 130(4): 254-259.
- Luzzatto L, Arese P (2018) Favism and Glucose-6-Phosphate dehydrogenase deficiency. N Engl J Med 378(1): 60-71.
- Dean L, Kane M, Victoria M Pratt (2020) Rasburicase Therapy and G6PD and CYB5R Genotype. Medical Genetics Summaries, National Center for Biotechnology Information.
- Luzzatto L, Ally M, Notaro R (2020) Glucose-6-phosphate dehydrogenase deficiency. Blood 136(11): 1225-1240.
- Domingo GJ, Advani N, Satyagraha AW, Sibley CH, Rowley E, et al. (2019) Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: challenges and opportunities. Int Health 11(1): 7-14.
- Adeyinka A, Bashir K (2022) Tumor Lysis syndrome. StatPearls.
- Wilson FP, Berns JS (2014) Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis 21(1): 18-26.
- Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, et al. (1991) The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 324(6): 377-384.
- Belfield KD, Tichy EM (2018) Review and drug therapy implications of glucose-6-phosphate dehydrogenase deficiency. Am J Health Syst Pharm 75(3): 97-104.
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, et al. (1995) Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 274(1): 29-34.
- Patel KK, Brown TJ, Gupta A, Roberts T, Marley E, et al. (2019) Decreasing Inappropriate Use of Rasburicase to Promote Cost-Effective Care. J Oncol Pract 15(2): e178-e186.
-
Lorie Ann Foster*, Nancy Lin, Ki Hong Oh, Anna Andrzejczyk and Alison T Stopeck. Rasburicase Induced Hemolysis in A Patient with Undiagnosed Glucose 6 Phosphate Dehydrogenase (G6PD) Deficiency. Arch Clin Case Stud. 3(5): 2024. ACCS. MS.ID.000571.
-
Glucose 6 phosphate dehydrogenase (G6PD); hematologic; lymphoma; chronic kidney; chemotherapy; scleral icterus; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






