 Case Report
Case Report
Diagnosis And Treatment of Cerebral Proliferative Angiopathy: Case Report
Balderas-Chairez AT1*, Velázquez-Luis JM1, Hernández-Gurrola MA1, Arellano-Sotelo J1, Balderas-Martinez A2, Sariñana-Navarrete KP3 and Avelar-Garnica FJ1
1Radiology and Imaging Department, Unidad Médica de Alta Especialidad, Hospital de Especialidades “Dr. Bernardo Sepúlveda Gutiérrez”, IMSS, Ciudad de México, México
2Anesthesiology Department, Hospital General de Durango, Secretaria de Salud, Durango, Mexoico
3Anesthesiology Department, Hospital General de Zona, IMSS, Durango, Mexico
Andres Tlacaelel Balderas Chairez, Radiology and Imaging Department, Unidad Médica de Alta Especialidad, Hospital de Especialidades “Dr. Bernardo Sepúlveda Gutiérrez”, IMSS, Ciudad de México, Mexico
Received Date:January 09, 2024; Published Date:January 18, 2024
Abstract
Cerebral proliferative angiopathy (CPA) is an uncommon condition characterized by widespread intravascular shunting. It is crucial to distinguish it from cerebral arteriovenous malformations. This case report focuses on a patient with cerebral proliferative angiopathy, illustrating a well-documented and progressive emergence of hyper vascular shunting extensively affecting the right hemisphere, along with its treatment. The patient’s condition was supported by angiographic and tomographic findings. This case represents the initial documentation of the evolution of cerebral proliferative angiopathy and its corresponding treatment.
Keywords:Cerebral proliferative angiopathy; arteriovenous malformations; cerebral angiography
Abbreviations:CPA: Cerebral Proliferative Angiopathy; MRI: Magnetic Resonance Imaging; MRA: Magnetic Resonance Angiography; AVMs: Arteriovenous Malformations; SVD: Small Vessel Disease
Introduction
CPA is a complex vascular disorder characterized by abnormal angiogenesis within the central nervous system. While historically perceived as a rare condition, CPA has gained increased attention due to its association with various neurological manifestations. A comprehensive understanding of the underlying pathophysiology and the identification of contributing factors are essential for accurate diagnosis and effective management [1]. CPA is an extremely rare progressive angiopathy with fewer than 100 reported cases in the literature. CPA accounts for about 3.4% of all cerebral AVMs [2]. Formerly known as diffuse nidus type or holohemispheric giant cerebral AVM [3], CPA most commonly affects adolescent and middle-aged women. Presenting symptoms include seizures, headache, and transient ischemic attack. Diagnosing cerebral proliferative angiopathy poses a substantial challenge, given the absence of a singular diagnostic test that can conclusively confirm its presence. Neuroimaging techniques play a pivotal role in the evaluation of CPA, with magnetic resonance imaging (MRI) offering visualization of characteristic features such as microangiopathic hemorrhages and subcortical ischemic lesions [4].
Advancements in imaging modalities, including magnetic resonance angiography (MRA), have significantly enhanced the ability to detect vascular proliferation and provide detailed insights into the morphology of affected vessels [5]. The presence of diffuse cerebral vessel involvement, lack of dominant venous drainage, and presence of normal brain parenchyma in between abnormal vessels are the key features that differentiate this entity from AVM. In summary, cerebral proliferative angiopathy emerges as a diagnostic and therapeutic enigma in clinical practice. As a more profound understanding of its underlying mechanisms is achieved, new opportunities for early identification, targeted interventions, and the development of preventive strategies are anticipated [6]. This report seeks to provide a comprehensive exploration of cerebral proliferative angiopathy, underscoring the critical importance of clinical recognition and delineating potential future research directions to enhance the overall management of this intricate pathology.
Case Report
A 70-year-old female with no significant medical history began experiencing symptoms in July 2023, with oppressive-type headaches rated 8 out of 10 on the EVA scale, occurring daily. These headaches were accompanied by nausea, dysarthria, and weakness on the left side of the body, leading to walking with support. An Angio CT was performed, revealing a vascular malformation in the right frontal lobe. The malformation receives arterial supply from the middle and anterior cerebral arteries, with venous drainage and surface involvement (Figure 1). Subsequently, a cerebral angiography was performed, revealing a vascular malformation extending into the right frontal lobe. This lesion presented a diffuse nidus with approximate dimensions of 81 x 74 x 47 mm in its cephalocaudal, anteroposterior, and transverse axes, respectively. It received supply through multiple feeding branches, mainly from the pre- and post-communicating segments of both anterior cerebral arteries, cortical segments of the right middle cerebral artery, and the left posterior communicating artery. These vessels appeared moderately ecstatic. The malformation exhibited deep venous drainage to internal cerebral veins and the Galenic vein, as well as superficial venous drainage to the superior sagittal sinus, transverse sinuses, and sigmoid sinus (Figure 2). As part of the treatment, a right frontal craniotomy was performed, involving the resection of the lesion and the placement of multiple definitive clips. Subsequent postoperative monitoring was conducted using Angio tomography (Figure 3).
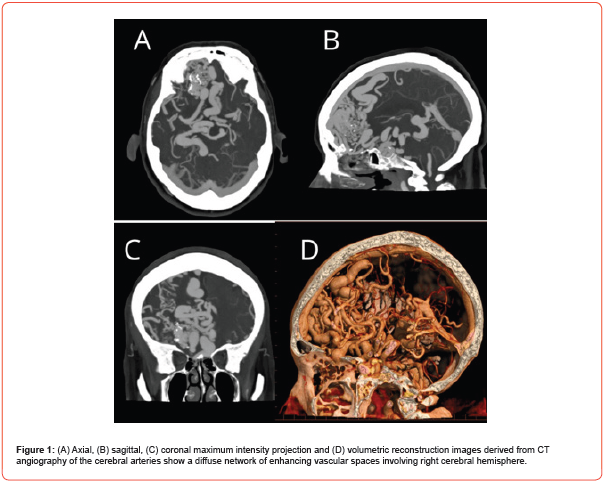
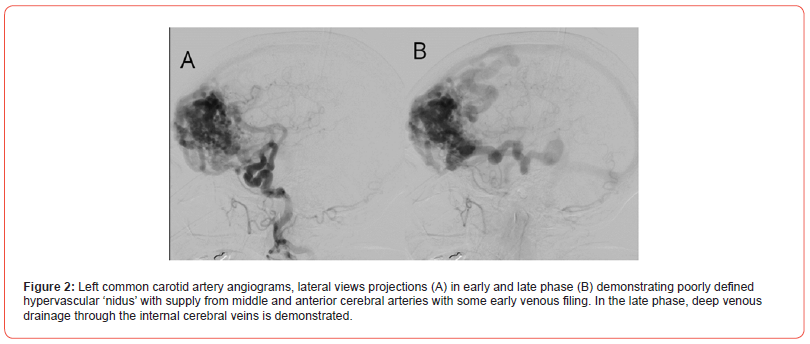

Discussion
Cerebral proliferative angiopathy (CPA) is a complex cerebrovascular disorder characterized by abnormal angiogenesis in the central nervous system. The pathology is closely associated with the deposition of amyloid protein, referred to as cerebral amyloid angiopathy (CAA). Amyloid deposition contributes to vascular fragility and abnormal vessel proliferation, initiating the cascade leading to CPA. Understanding these pathophysiological mechanisms is fundamental for targeted therapeutic interventions [7]. Clinical presentations of CPA vary widely, ranging from asymptomatic cases to severe manifestations such as cerebral ischemic events or intracerebral hemorrhages. The diverse spectrum of clinical manifestations poses challenges in timely recognition and diagnosis, requiring an appreciation of the heterogeneity of symptoms for early intervention [8]. Diagnosing CPA is a complex task due to its varied presentations. Advanced neuroimaging techniques, such as magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA), play a pivotal role in visualizing characteristic features like microangiopathic hemorrhages and subcortical ischemic lesions. These imaging modalities provide crucial insights into the extent and nature of vascular abnormalities associated with CPA, aiding in diagnosis and treatment planning.
Despite advancements in neuroimaging, the lack of a singular gold standard diagnostic test for CPA remains a significant challenge. The diversity of clinical presentations and the absence of a definitive biomarker complicate the diagnostic process. Continued research efforts are needed to identify reliable diagnostic markers and establish a gold standard that can streamline the diagnostic pathway for CPA. Therapeutic strategies for CPA primarily focus on managing complications, such as intracerebral hemorrhage. However, there is currently no targeted treatment to halt the abnormal angiogenesis associated with CPA. The absence of specific therapeutic interventions highlights the need for innovative approaches to address the underlying pathophysiology and prevent disease progression. Future research should explore potential pharmacological targets and assess the efficacy of emerging treatment modalities. The association of CPA with small vessel disease (SVD) places it within a broader context of cerebrovascular disorders. Understanding the implications of CPA within the spectrum of SVD contributes to a holistic comprehension of cerebrovascular pathology. Shared pathophysiological mechanisms across different small vessel diseases may provide insights into common therapeutic targets [9].
Conclusion
In conclusion, CPA poses diagnostic and therapeutic challenges, warranting collaborative efforts across disciplines. Advances in neuroimaging have improved diagnostic capabilities, but the lack of a gold standard underscores the need for ongoing research. Future directions should prioritize the identification of biomarkers, the development of targeted therapeutic interventions, and the exploration of preventive strategies to address the underlying pathophysiology of CPA. This discussion serves as a foundation for ongoing research endeavors, emphasizing the complexity of CPA and the imperative to bridge diagnostic and therapeutic gaps in its management. Collaborative research efforts will contribute to a more comprehensive understanding of CPA and enhance clinical approaches to improve patient outcomes.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Acknowledgement
The workers at the Imaging Department collaborated on the execution of the procedures.
Conflicts of Interest
The authors of this manuscript have no conflicts of interest to disclose.
References
- Wardlaw JM, Smith C, Dichgans M (2019) Small vessel disease: Mechanisms and clinical implications. Lancet Neurol 18(7): 684-696.
- Lasjaunias PL, Landrieu P, Rodesch G (2008) Cerebral proliferative angiopathy: clinical and angiographic description of an entity different from cerebral AVMs. Stroke 39(3): 878-885.
- Liu P, Lv X, Lv M, Li Y (2016) Cerebral proliferative angiopathy: clinical, angiographic features and literature review. Interv Neuroradiol 22(1): 101-107.
- Biffi A, Greenberg SM, Jagiella JM (2011) Genetic risk factors for intracerebral hemorrhage: Insights from studies of patients with lobar hemorrhage. Stroke 42(1 Suppl): S5-S7.
- Fazekas F, Kleinert R, Roob G (1999) Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 20(4): 637-642.
- Knudsen KA, Rosand J, Karluk D, Greenberg SM (2001) Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 56(4): 537-539.
- Charidimou A, Boulouis G, Roongpiboonsopit D (2017) Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: a prospective study. Neurology 89(21): 2128-2135.
- Greenberg SM, Vonsattel JP, Stakes JW, Gruber M (1993) The clinical spectrum of cerebral amyloid angiopathy: Presentations without lobar hemorrhage. Neurology 43(10): 2073-2079.
- Panthi S, Khanal N, Poudel S (2022) Diffuse proliferative cerebral angiopathy: a case report and literature review on a very rare and misdiagnosed entity. J Surg Case Rep 2022(1): 620.
-
R Nhiri, S Bellamqddam, S Amaqdouf , N Elouafi. Cardiotoxicity: An Unusual Case of Methotrexate Overdose. 3(4): 2024. ACCS. MS.ID.000568.
-
Rheumatoid polyarthritis, Hypertensive, Diabetic, Anti-inflammatory drugs, Pancytopenia, Methotrexate.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






