 Editorial
Editorial
The Layered Leeway-Alveolar Rhabdomyosarcoma
Anubha Bajaj, Department of Biomedical sciences, India.
Received Date: May 24, 2023; Published Date: June 22, 2023
Editorial
Alveolar rhabdomyosarcoma is a soft tissue, mesenchymal neoplasm demonstrating a cellular lineage preponderantly of skeletal muscle cells. Alveolar rhabdomyosarcoma characteristically delineates cogent genetic fusion proteins wherein around ~60% neoplasms delineate PAX3-FOXO1 fusion gene and nearly 20% tumours exhibit PAX7-FOXO1 fusion gene. An estimated 20% of tumours are devoid of pertinent genetic fusions.
Alveolar rhabdomyosarcoma configures ~ 30% of rhabdomyosarcomas and nearly 1% of paediatric malignancies. Alveolar rhabdomyosarcoma commonly incriminates head and neck region, male urogenital tract, female urogenital tract, torso and upper or lower extremity.
Alveolar rhabdomyosarcoma is posited to emerge from precursor cells confined to skeletal muscle. Additionally, diverse genetic recombinant manifestations engender fusion proteins which dysregulate transcription and contribute functionally as an oncogene (1,2). Tumour cells depict a characteristic chromosomal translocation t (2;13) (q35; q14). The majority (~85%) of neoplasms express PAX3-FKHR or PAX3-FOXO1 genetic fusion. Chromosomal translocation t (1;13) (p36; q14) is encountered. Few (~20%) instances depict PAX7-FKHR genomic fusion. Absence of PAX/FOX1 fusion (20%) is associated with neoplasms demonstrating a comprehensively solid histological subtype. Aggressive tumours (~50%) delineate amplification of N-myc. Alveolar rhabdomyosarcoma predominantly arises as a sporadic neoplasm. A definitive genetic predisposition is absent (1,2).
Alveolar rhabdomyosarcoma commonly emerges within young adolescent subjects although no age of disease emergence is exempt. Primary tumour frequently represents a painless soft tissue mass. Tumefaction may induce pressure symptoms upon anatomic structures abutting the primary site of tumour emergence. An estimated 30% neoplasms demonstrate metastases upon initial tumour detection, especially within sites such as bone marrow, bones or distant lymph nodes. Tumours depicting PAX3- FOXO1 genetic fusion are predominantly encountered within older children or young adults (1,2). Grossly, a fleshy, tan or grey tumefaction is encountered.
Upon cytological examination, a uniformly cellular tumour is composed predominantly of multinucleated giant cells and exhibits budding alveolar tumour pattern. Upon microscopy, miniature tumour cells appear incorporated with minimal cytoplasm. Tumour cell nuclei appear spherical and pervaded with normal, dull, chromatin structures (2,3).
Alveolar rhabdomyosarcoma exemplifies distinct variants as
• classical subtype of alveolar rhabdomyosarcoma is comprised of nests of neoplastic cells configuring alveolar spaces with tumour cells adhering to periphery of alveoli. Characteristically, a hobnail or tombstone appearance or noncohesive papillary articulations may be observed. Non cohesive tumour cells appear to float within the centric zone of alveolar spaces. Tumour cell nuclei are disseminated peripherally and enunciate a wreath-like appearance. Multinucleated giant cells may be discerned. Normal skeletal muscle fibres appear entrapped within tumour zones.
• solid subtype is composed of sheets of neoplastic cells or tumour cell nests segregated by attenuated fibro-vascular septa. Alveolar articulations appear absent. Tumour cells and nuclei delineate features of anaplasia. An estimated 30% of tumours depict occasional rhabdomyoblasts. Foci of coagulative tumour cell necrosis are frequently discerned. Upon ultrastructural examination, tumour cells appear immune reactive to biomarkers of skeletal muscle differentiation (2,3) (Figure 1,2).
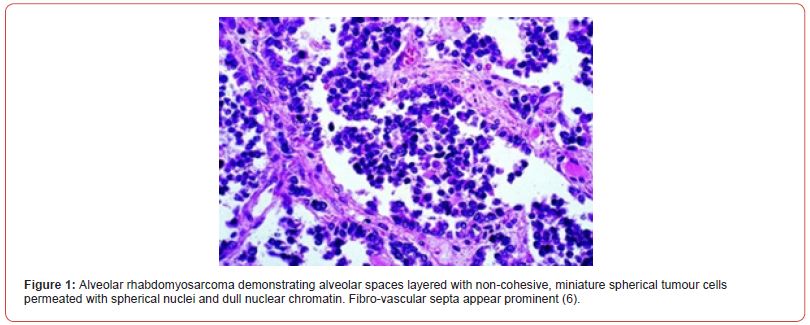
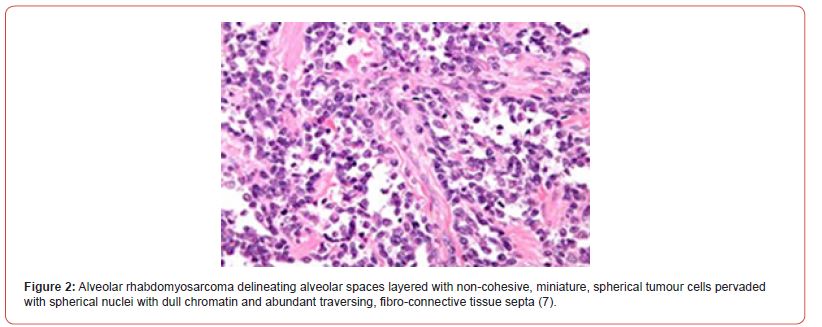
Clinical group (CG) of rhabdomyosarcoma as denominated by Intergroup Rhabdomyosarcoma Study Group (IRSG) categorizes rhabdomyosarcoma as
• stage I: Tumour confined to site of origin as ~localized tumour, anatomically confined to site of origin wherein tumour is amenable to complete surgical resection ~localized tumour with infiltration into adjacent anatomical structures wherein tumour is amenable to complete surgical resection.
• stage II: Tumour with localized infiltration as ~localized tumour with possible gross or total surgical resection accompanied by probable persistence of microscopic residual disease ~locally extensive tumour with regional lymph nodes metastasis wherein tumour is amenable to complete surgical resection ~locally extensive tumour with regional lymph node metastasis wherein tumour is amenable to complete surgical resection. However, microscopic residual disease may be discerned.
• stage III: Tumour is localized and extensive as ~localized extensive tumour with occurrence of gross residual disease upon surgical tissue sampling ~localized extensive tumour with occurrence of gross residual disease following extensive tumour resection with ≥ 50% of tumour de-bulking •stage IV: metastatic rhabdomyosarcoma as ~primary tumour of variable magnitude along with or absence of incriminated regional lymph nodes and distant metastases (3,4).
TNM staging of Rhabdomyosarcoma is designated as
• stage I: Tumour is confined to a region with favourable prognosis ~Tumour of variable magnitude with localized invasion into adjacent areas along with or devoid of regional lymph node metastasis ~Tumour with distant metastasis
• stage II: Tumour is confined to a region with unfavorable prognosis ~Tumour magnitude ≤ 5 centimetres with absent local invasion into adjacent anatomical structures along with or devoid of regional lymph node metastasis or distant metastasis
• stage III: Tumour confined to a region with unfavorable prognosis ~Tumour magnitude ≤ 5 centimetres with regional lymph node metastasis OR Tumour magnitude > 5 centimetres along with or devoid of regional lymph node metastasis. Distant metastasis is absent •stage IV: Tumour of variable magnitude which may commence at diverse favourable or unfavorable sites. Distant metastasis is present (3,4).
Favourable sites of tumour emergence are designated as biliary tract, orbit, head and neck excluding para-meningeal region and genitourinary tract excluding prostate and urinary bladder. Apart from aforementioned sites, remaining sites of tumour emergence are denominated as unfavorable sites (3,4).
Risk stratification of rhabdomyosarcoma is contingent to factors such as clinical group, site and magnitude of tumour, age of incriminated subject, histological features, associated metastases and status of regional lymph nodes. Alveolar rhabdomyosarcoma encountered in subjects demonstrating stage I, stage II or stage III disease with clinical stage I to clinical stage III and clinical group 1 to clinical group 3 are designated as tumours with intermediate risk. Alveolar rhabdomyosarcoma occurring in subjects with stage IV disease exemplifying clinical stage IV and clinical group 4 are denominated as high-risk neoplasms (3,4).
Alveolar rhabdomyosarcoma is immune reactive to myogenin, desmin, muscle specific actin, MyoD1 or P-cadherin. Besides, tumefaction is immune reactive to anaplastic lymphoma kinase 1(ALK1), CAM5.2, wide spectrum cytokeratin, neuroendocrine markers, p80 or PAX5. Alveolar rhabdomyosarcoma requires segregation from neoplasms such as alveolar soft part sarcoma, Merkel cell carcinoma or metastatic neuroendocrine carcinoma (4,5). Alveolar rhabdomyosarcoma can be appropriately treated with standardized surgical procedures, radiation therapy and intensive chemotherapy. Tumours lacking genetic fusion proteins and demonstrating low risk clinical features may be subjected to decimated modes of therapy (4,5). In contrast to embryonal subtype, alveolar rhabdomyosarcoma is associated with overall inferior prognostic outcomes. Tumour staging adopted in concurrence with Intergroup Rhabdomyosarcoma Study Group appears predictive of prognostic outcomes (4,5). Metastatic alveolar rhabdomyosarcoma with PAX3-FOXO1 fusion proteins delineates an adverse prognosis. Additionally, variables influencing overall survival emerge as ~anaplastic features upon morphological assessment ~site and magnitude of primary tumour ~proportionate localized tumour invasion ~quantifiable incriminated distal lymph nodes ~distant metastases into various sites (4,5).
Acknowledgement
None.
Conflicts of Interest
No conflicts of interest.
References
- Liang X, Wang S, Li T, Longlong Liu, Yu Duan, et al. (2022) Alveolar rhabdomyosarcoma of epididymis: A case report. Front Oncol 12 :1027504.
- Zhang Y, Huang W, Li L, Yongkang Qiu, Hao Jiao, et al. (2022) Retroperitoneal alveolar rhabdomyosarcoma intruding into spinal canal: A case report and literature review. Front Med (Lausanne) 9:1019964.
- Kaseb H, Kuhn J, Babiker HM. Stat Pearls International 2022, Treasure Island, Florida.
- Gallego S, Chi YY, De Salvo GL, Minjie Li, Johannes HM Merks, et al. (2021) European paediatric soft tissue sarcoma Study Group and Children's Oncology Group. Alveolar rhabdomyosarcoma with regional nodal involvement: Results of a combined analysis from two cooperative groups. Pediatr Blood Cancer 68(3): e28832.
- Makimoto A (2022) Optimizing Rhabdomyosarcoma Treatment in Adolescents and Young Adults. Cancers (Basel) 14(9): 2270.
- Image 1 Courtesy: Nature
- Image 2 Courtesy: Libre Pathology
-
Anubha Bajaj*. The Layered Leeway-Alveolar Rhabdomyosarcoma. Arch Biomed Eng & Biotechnol. 7(3): 2023. ABEB. MS.ID.000663.
-
Bone marrow, Distant lymph nodes, Tumours, Normal, Dull, Chromatin structures, Cytoplasm, Alveolar, Rhabdomyosarcoma, Articulations, Amenable, Microscopic
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






