 Review Article
Review Article
Potential Replacement of Pentaeritritol Tetranitrate Explosive Aggregate Polymer (Petn): A Short Review
Pighinelli L*, Dias PSR, Paz LR, Guimaraes, FM, Broqua JS and Lisboa CM
Biomatter P&D and Innovation of biomaterials Lab Av Bento Goncalves, Brazil
Pighinelli L, Biomatter P&D and Innovation of biomaterials Lab Av Bento Goncalves, Brazil.
Received Date:September 16, 2019; Published Date:September 24, 2019
Abstract
The use of natural polymers as substitutes for synthetic polymers in the development of aggregating materials for the pentaerythritol tetranitrate (PETN), explosive is a poorly explored area, but with the ability to enable the development of new sustainable, more chemical friendly and economically, using as an alternative a renewable material like chitosan and its derivatives. Chitin, a natural polymer that makes up the exoskeleton of crustaceans, insects, and the fungal cell wall, along with their derivatives, such as chitosan, offer attractive chemical, physical, and biological properties. It is noteworthy that chitin is found in high abundance in nature, and allows obtaining materials capable of offering interesting characteristics, such as chelating capacity, biocompatibility, biodegradability, nontoxic [3]. In this review, is possible to see the possibility to use a natural polymers as a potential materials to use as na aggregate polymer in PETN, as well as the increasing trend of the use of renewable natural polymers to develop creation of materials with high technological grade for mant areas of application. The aim of this study is to show the potential of using chitosan as an aggregator polymer for PETN crystals.
Introduction
Pentaerythritol tetranitrate (PETN) is an explosive that exhibits the functionality of the ester nitrate group. The explosive capacity and high chemical stability led to large-scale production of PETN, which could only be achieved after pentaerythritol production, that is the raw material for its production. PETN was the first explosive to be prepared by pentaerythritol nitration in 1894. Its commercial production was achieved after the consolidation of the synthetic routes of formaldehyde and acetaldehyde, precursor compounds for the synthesis of pentaerythritol [1]. This explosive has military, industrial, medical and civil applications due to its high energy density, which is strongly linked to particle size and structure morphology, such as crystal lattice defects, surface area and structural phases [2,3]. During applications, formulations containing PETN are stabilized by additives that increase handling safety. In the case of plastic explosives, such as those in the C-4 family, for example, it is common for petroleum-derived synthetic polymers to be used for this purpose [3-5]. A natural polymer that comes to the attention of researchers is chitosan and its derivatives, which is obtained by the deacetylation of chitin, which came from fishing industry waste. It is a linear, cationic polysaccharide with protonation (addition of protons) of the amino group NH3+ (NAIR, R.S.,2019). Due to its characteristic of presenting free amino groups, chitosan has the capacity to react with several molecules, making the biopolymer with greater availability of groups pending cites chitosan among the most studied natural polymers [37]. Due to the increasing employability of natural polymers to obtain materials with high technological grade, which can be easily used in different areas, such military, engineering and health for example. The focus of this review is to evaluate the possibility of using chitosan and its derivatives as an aggregating polymer for PETN crystals, increasing the high technological grade for many diverse areas of application
Structure and Properties of Pentaeritritol Tetranitrate (Petn)
Pentaerythritol tetranitrate, also known as erythrin tetranitrate or simply called PETN (C5H8N4O12) is one of the most popular explosives in the world due to its military application, use in mining, construction and medicine. PETN has a relative effectiveness factor, defined as the relative mass of trinitrotoluene (TNT) to which an explosive is equivalent, 1.66. It is more sensitive to shock or friction than TNT. Its use is basically as a potentiator, that is, PETN is commonly used in conjunction with other compounds in explosive charges. As an example, we can mention Semtex, an explosive plastic based on PETN and RDX [4,6,7]. The chemical structure of PETN (Figure 1).
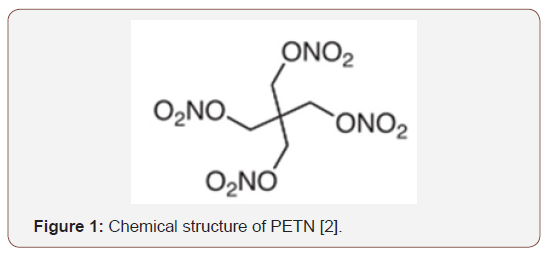
It use as mentioned, is present in several areas, such as development of explosives for military use, in mining to provide mine detonation for the extraction of mineral resources, in medicine, where it can act as a vasodilator and in civil construction for destruction of structures [4,8]. Pentaerythritol tetranitrate is an organic ester nitrate, and as such its synthesis takes place from an alcohol or polyol, and when obtained it is solid in the form of crystals and has an irritating smell. Its characteristics generally resemble TNT [5,9].
Summary of pentaeritritol tetranitrate
There are two routes for PETN synthesis: sulfonitric and nitrated. The mixture of nitric and sulfuric acids makes up the most common and most economically viable agent used in the nitrated route. This route generates many byproducts because sulfuric acid is not characterized as a good solvent for many organic substrates. Despite the generation of byproducts, this route plays an important role in the direct action in the production of nitrate esters, at both industrial and laboratory levels. With respect to nitric acid used as a reagent in esterification (or nitration) reactions, this is the essential mechanism for obtaining parental alcohol, which is considered a good solvent for organic substrates, besides having a high solubility in polyols [6,10,11].
Solid polyols such as pentaerythritol, erythritol and mannitol are commonly nitrated with nitric acid. The procedure of bubbling dry air through nitric acid to remove nitrogen oxides that may be present in the mixture is present, followed by the addition of a trace of urea to remove nitrous acid that may have been formed throughout. of the process. The mixture is cooled to approximately 0°C and excess acid is added, keeping the system stirring for a short time. Thereafter, the solution is poured into excess water and subsequently the nitrate ester is extracted or filtered, yielding a good yield in the end. In such cases, the use of excess nitric acid is essential to ensure complete nitration of the substrate. The yield obtained from the method described is close to 95% [6,12]. Figure 2, shown below, shows the synthesis reaction of PETN (Figure 2).
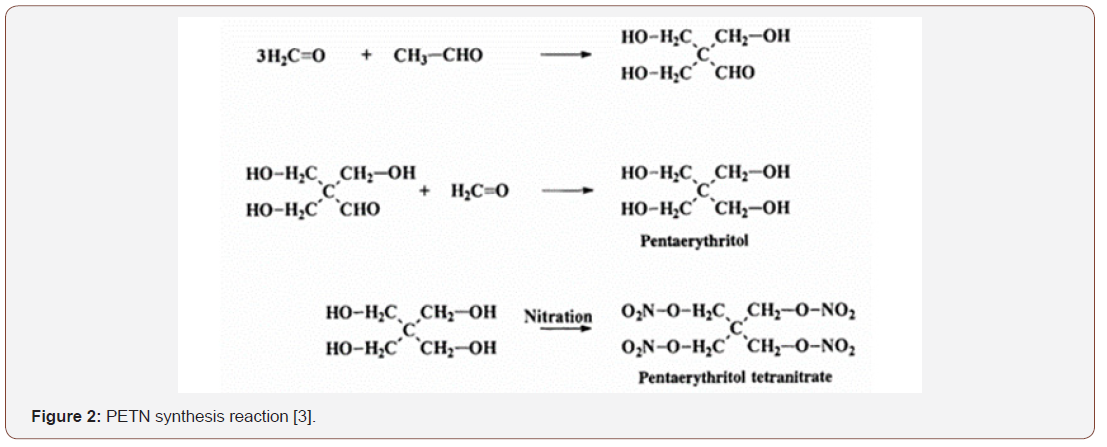
Pentaeritritol tetranitrate properties
Following are the physical, chemical, thermochemical and explosive properties of PETN.
Physical properties: Melting point: 141°C; Solubility: Insoluble in H₂O, H₂SO₄ conc., HNO₃ below 30%; Soluble: Organic solvents (acetone) above 92% at 50°C; Specific heat: 0,4 cal / g / °C; Specific mass: 1.77 g /cmᵌ; Loading density: 1.51 g /cmᵌ to 1.773 g /cmᵌ [7,13].
Chemical and thermochemical properties: Not decomposed by Na₂SO₃ at 50°C; 25% NaOH causes slight decomposition; Boiling 20% FeCl₂ completely decomposes; undergoes hydrolysis by H₂O at 100°C; Autoclaved at 125°C releases HNO₃, forming pentaerythritol trinitrate; High chemical stability, depending on the symmetry of the molecule, resisting for 20 months at 65°C; does not dry react with: copper, brass, aluminum, stainless steel, nickel, magnesium, zinc and cadmium; Nitrogen content: 17.7%; Oxygen Balance: -10.1%; Formation Heat: 128.7 Kcal / mol or 390 Kcal / kg; Heat of combustion: 618,7 Kcal / mol [7,13,14].
Explosivity properties: Detonates when heated between 200 and 205°C; May detonate under intense U.V. Radiation; Shock sensitivity to 2 kg weight: 15 cm; Little sensitive to friction but should be agglomerated with wax; Detonation speed: 1,530 Kcal / Kg; Burst Temperature: 4,230 °C [7].
PETN Aggregating Polymers
PETN, like some other explosive initiators, has high sensitivity, which often implies the need to use a polymeric matrix that enables the reduction of this characteristic, providing a safer handling. Among the matrices employed as PETN crystal aggregating agents, we can cite as examples: Poly (ethyl acrylate) with dibutyl phthalate, silicone rubber, epoxy diethylenetriamine resin (DETA), polytetrafluoroethylene - PTFE (Teflon), polyamide (Nylon), among others. The main aggregating polymers employed with PETN, as well as the description of their main characteristics and applications are presented below.
Poly (Ethyl Acrylate) with Dibutil Ftalate
They are reactive, crosslinked, high-strength adhesive monomers and structural, generally hardened as elastomeric polymers as part of the formulation [8,15]. Typically, when employed in explosive mixtures, these polymers are used alone or copolymerized with a variety of other polymers in different particle size classes [9, 16]. Mixtures derived from this material with explosives contain one or more explosive substances, as well as additives, binders, plasticizers, stabilizers, flegmatizers and the like. Additives and binders are relevant as they provide the appropriate mechanical properties and the ability to operate through various technologies such as pressing, extrusion and casting. This material also guarantees a reduction in the sensitivity of explosives, improving their sensitivity to impacts and friction, ensuring the protection of crystals against external environmental factors [7,9,17].
Silicone rubber
It is a class of materials that can take the form of drying adhesives, pasty materials, fluidized adhesives, among others, and are characterized by excellent weathering resistance, external temperatures, as well as the ability to accommodate substrate movement [11,16,18]. For formulations with explosives, there are numerous applications with silicone, which can act as a drying agent that does not bind to all substrates, favoring the development of new combinations, allowing modification of the desired characteristics [12,19]. The sealing properties and the ability to accommodate substrate movement make it possible to obtain adequate physical properties, which enable structural applications that require high movement capacity [14,20].
Epoxydylethylenetriamine resin (Deta)
Epoxy resins are characterized as capable of forming crosslinked matrices with excellent adhesion to a wide variety of substrates, making them suitable for adhesive applications where high strength under adverse conditions is required. Its properties include negligible shrinkage during curing, excellent chemical resistance, ability to bond non-porous substrates and great versatility, as well as molecular weight ranges, as well as a variety of sizes [14,19]. Epoxy resin adhesives are commonly used in concrete and metal structure applications. Its electrical properties coupled with durability favor potting and encapsulation processes [16,20]. In general, DETA compounds provide good cure at room temperature when presented in appropriate stoichiometric proportions [12,21].
Tetrafluoroethylene poly - PTFE (Teflon)
Polytetrafluoroethylene-PTFE (Teflon) is a high molecular weight polymer that favors insolubility in commonly used solvents, influences chemical inertia, even under external reaction conditions, contributes to increased melting point, viscosity, heat resistance coefficient of friction over a wide temperature range [22-24]. Teflon can be used in electrical, microelectronic, mechanical and chemical applications such as seals and piston rings and coated glass, which demonstrates high mechanical strength. PTFE powder has a lower molecular weight, which also allows its use as additives in plastics, paints and lubricants [25,26]. PTFE is hydrophobic in character and reduces solubility when present in low melting mixtures, has good long-term stability, and is a material widely used in sensitive applications in the areas of space aviation and nuclear technology [27,28].
Polyamide (nylon)
Polyamides are polymers with repeating units that incorporate an open current or closed ring, and are characterized by excellent mechanical and electrical properties, as well as high thermal stability that guarantees good heat resistance, a feature that favors their use in molds. of films and varnishes, for example [16,29,30]. Polyamides in advanced technologies for making insulating films, coatings and laminates, molded parts, structural adhesives, insulation foams, fibers, composites and permeable membranes [31].
Polyamides are not necessarily polymerized from the same substance; however, they depart from the amide functional group, which directly influences the properties, the shorter the distance between these groups, the better their mechanical and thermal properties. Among the important factors of polyamides are the degree of crystallinity and water absorption capacity, which in turn acts as a natural plasticizer. The higher the crystallinity, there are improvements in the tensile strength, stiffness and creep of the material, resulting in high hardness and abrasion resistance. However, if the degree of crystallinity is reduced, the absorption of moisture by the structure will increase, as well as having higher toughness and impact resistance [13,31].
Chitosan - Structure, Properties and Applications
Chitosan (poly (β- (1,4) -D-glucosamine), the main chitin derivative in terms of applications, is a polymer obtained from the chemical or biological treatment of fishery tailings which, after treatment, can be used to obtain biodegradable, non-toxic, biocompatible, bactericidal, bioactive and anti-inflammatory biomaterials for applications in the most diverse areas, including regenerative medicine, water and wastewater treatment, pharmaceutical, cosmetic, toxicological, food industry, among others. Figure 3 shows the chemical structures of chitin and chitosan [32] (Figure 3).
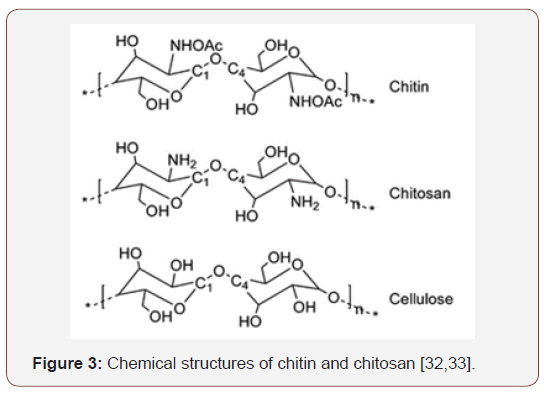
The amino groups of the chitosan structure give the molecule cationic character, making the material characterized in acid medium as a linear positively charged polyelectrolyte that can Interact with negatively charged surfaces such as anionic polysaccharides, proteins and other structures [34]. According to the literature, chitosan is soluble in dilute acid medium, and due to the positive character of the molecule, the structure is susceptible to the addition of protons (protonation) in the amino groups (NH3), which give the excellent properties mentioned above. material. From the solubilization of chitosan, it is possible to obtain samples in the form of gels, films and fibers for various uses [35]. With regard to chitosan production, it is economically viable and environmentally friendly due to the large amount of waste from the fish industry, which, instead of causing damage to the environment, has its potential applied in the development of high technology biomaterials [36]. From chitosan in powder form, for example, it is possible to obtain nanocrystalline chitosan, modified form of chitosan, through different methods such as: chitosan salt coagulation, inotropic gelation, microemulsion, polyelectrolyte complexation and emulsification/solvent diffusion [37]. Nanocrystalline chitosan is characterized by the special properties of the initial chitosan, which indicates that the processes for obtaining it do not interfere with the essential characteristics of this polymer. This form of chitosan stands out for its use in medical applications, drug transport systems, water and effluent treatment, etc. [38].
Chitosan as a potential PETN aggregate polymer
Chitosan is a highly versatile natural polymer that behaves like a cationic polyelectrolyte that has ion-exchange properties, as already mentioned. Its free amino groups, which give the structure a positive character, offer the possibility of investigating its potential as an aggregating polymer for pentaerythritol tetranitrate. In the work entitled “Adapting the Sensitivity of Starting Explosives” by Manner et al. [2], shows the sensitivity properties of the manipulation of PETN derivatives with their respective structures are related, as well as the role of the central carbon-linked functional group of PETN with regard to oxygen balance, thermal stability, impact sensitivity, frictional resistance and potential for hydrogen bonding. Among the PETN compounds obtained in the study are derivatives that have hydrogen, amine and methyl groups attached to the central carbon atom of the pentaerythritol tetranitrate structure in order to explore the effects on sensitivity management, oxygen balance and hydrogen bridges. The derivatives, including chitosan, obtained in the study by Manner et al. [2], are presented in (Figure 4).
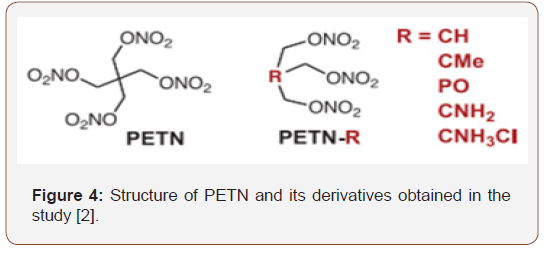
In obtaining PETN derivatives, according to Manner et al. [2], more than half were in the liquid state at room temperature, which can be seen through (Table 1).
Table 1:PETN derivatives and their respective physical states at room temperature [2].
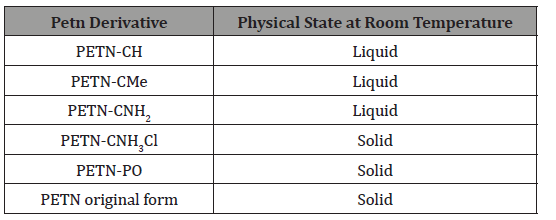
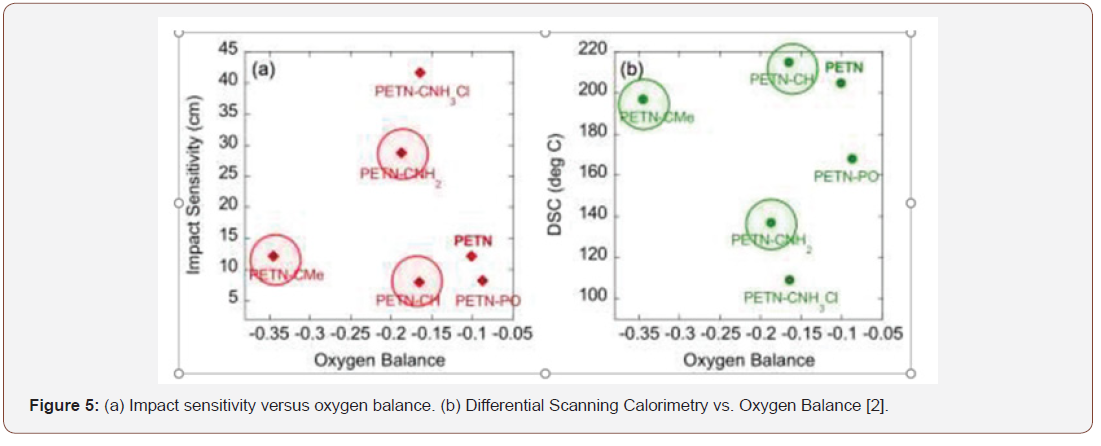
It was verified that the derivative of PETN-CNH2 presented one of the lowest thermal stability, which suggests lower resistance to temperature changes, which may cause alteration of its composition. Regarding the oxygen balance, as the missing (negative sign) or excess (positive sign) oxygen mass of this explosive to transform all carbon, if any, into carbon dioxide and all hydrogen, if present in water, divided by the molar mass of the explosive. It was found that oxygen balance and physical state of materials do not play an important role in handling and thermal stability. Figure 5 presents the values obtained in graphs that relate the impact sensitivity to oxygen balance and differential calorimetry scan with oxygen balance (Figure 5)
According to the literature [2], the central carbon-linked functional group of PETN is directly related to the impact sensitivity. An example is the amino group derivative (PETN-CNH2), which has the potential to form hydrogen bridges with nitro groups within the molecule and in neighboring molecules, a condition not found in the methyl group derivative (PETN). The potential for hydrogen bonding of the PETN-CNH2 derivative corresponds to the observed decreased impact sensitivity. The same behavior can be seen in the case of the PETN-CNH3Cl derivative, which is an amino-HCl salt that exhibits higher potential for intermolecular hydrogen bridges, resulting in significantly lower impact sensitivity compared to other PETN derivatives. Figure 6 illustrates the comparison with respect to the potential for hydrogen bonding of amino and methyl groups (Figure 6).
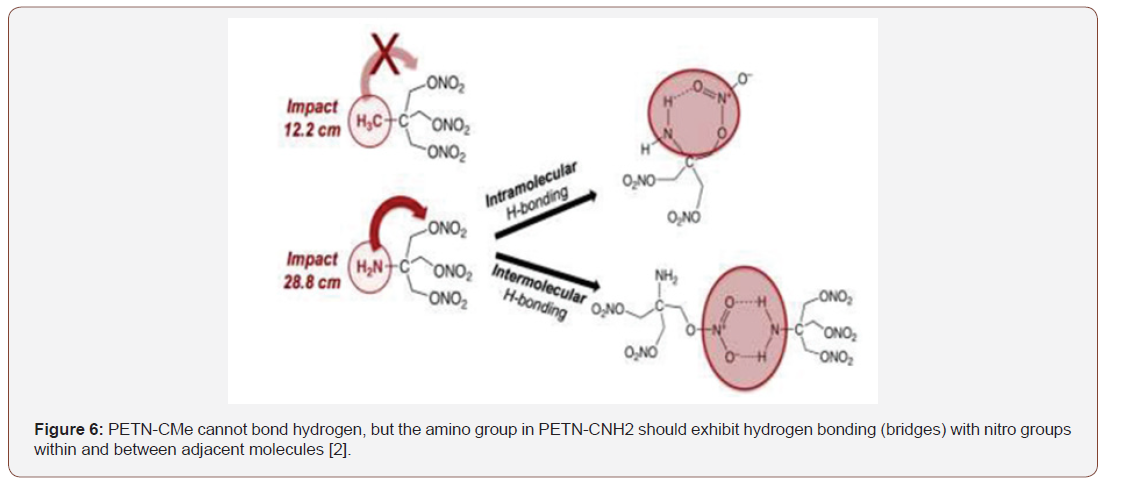
According to Manner et al., Preliminary results indicate that the ability to form hydrogen bonds within the molecule and molecular network significantly reduces impact sensitivity as well as increases frictional resistance. The literature review in this article allows us to investigate in order to evaluate the use of chitosan as an aggregator polymer of PETN as an opportunity to promote the use of natural polymers for this purpose, taking into account since in practice most polymers of synthetic origin are employed.
Final Considerations
From the present study it was possible to verify that the PETN, when used alone, presents high impact sensitivity and low resistance to friction, which results in the need to incorporate a polymeric matrix that allows to change such characteristics, allowing a safer handling. At the same time, there is a growing tendency of the use of natural polymers in the development of materials with high technological degree for the most diverse areas, which implies the intention to relate both exposed situations. As a proposal, it was considered the possibility of using chitosan for the elaboration of polymeric matrix to act as an aggregator polymer of pentaerythritol tetranitrate crystals. From a wide evaluation of studies related to the characteristics, properties and applications of the materials that make up the polymeric matrices commonly used together with PETN, it can be inferred that chitosan presents chemical and physical characteristics that favor its use for this purpose. According the literature, amino groups from chitosan, provide analogous characteristics with the synthetic polymers commonly used as a aggregator polymer when they are chemically linked to the central carbon of PETN. Among the similar characteristics are the reduction in impact sensitivity, increased frictional resistance and adhesion capacity, also promoted by matrices composed of poly (ethyl acrylate) with dibutyl phthalate, polyamide (Nylon)) and epoxy diethylenetriamine resin (DETA). Also, according to the literature, reduced impact sensitivity and increased resistance to friction, that are directly related to the ability of hydrogen bridges by the derived structures obtained. As a disadvantage of the structure in relation to silicone rubber, poly tetrafluoroethylene (PTFE) and polyamide (Nylon), for example, lower thermal stability may be mentioned, which implies less resistance to temperature changes. It is emphasized that such disadvantage can be mitigated by the use of additive materials, as well as the adaptation of the obtained technique.
The fundamental highlight of this study is that the potential use of chitosan for the development of polymeric matrix to aggregate PETN crystals is something innovative, that is, something that can bring the opportunity to generate significant changes, ensuring a new field. Chitosan application. It is noteworthy that the production of this natural polymer is economically viable and ecologically correct, as it favors that the waste from the fishing industry be converted into special materials, which have the potential to subsidize actions with the purpose of contributing to the generation of a new class of aggregating materials in the field of explosives
Acknowledgement
None.
Conflict of Interest
No Conflicts of Interest.
References
- Agrawal, Jai Prakash (2010) High Energy Materials: Propellants, Explosives and Pyrotechnics. Wiley‐VCH Verlag GmbH & Co. KGaA.
- Manner, Preston, Snyder, Dattelbaum, Tappan et al. (2015) Tailoring the Sensitivity of Initiating Explosives.
- Akhavan J (2011) The Chemistry of Explosives. Royal Society of Chemistry.
- Paul W Cooper (1996) Explosives Engineering, Published by Wiley VCH.
- Chambers D, Brackett C, Sparkman O (2002) Perspectives on Pentaerythritol Tetranitrate (PETN) Decomposition.
- Jimmie C Oxley, David Furman, Austin C Brown, Faina Dubnikova, James L Smith et al. (2017) Thermal Decomposition of Erythritol Tetranitrate: A Joint Experimental and Computational Study. J Phys Chem 121: 16145−16157.
- Bowen R, Argentar H (1973) A method for determining the optimum peroxide-to-amine ratio for self-curing resins. J Appl Polymer Sci 17(7); 2213-2222.
- Brendley Jr WH (1973) Fundamentals of acrylics. Paint and Varnish Production 19–27.
- Owen MJ, Klosowski JM, (1988) Adhesives, Sealants and Coatings for Space and Harsh Environments. Plenum Press, New York, p 283.
- Matyas R, Lycka A, Jira sko R, Jakovy Z, Maixner JM et al. (2016) Analytical Characterization of Erythritol Tetranitrate, an Improvised Explosive. J Forensic Sci 61(3): 759−764.
- Liles DT, Shephard NE (1992) Science and Technology of Building Seals, Sealants, Glazing and Waterproofing In: Klosowski JM (Ed,) American Society for Testing and Materials 2: p 1142, Philadelphia, USA.
- Klosowski JM, Gant GAL (1979) Plastic Mortars, Sealants, and Caulking Compounds. In: RB Seymour (Ed,) ACS Series 113, American Chemical Society p: 117, Washington DC.
- Lee H, Neville K (1967) Handbook of Epoxy Resins. McGraw-Hill, New York.
- Avakian P, Starkweather H, Fontanella JJ, Wintersgill MC (1993) Polym Mater Sci Eng 70: 439.
- Zybin SVL, Goddard WA, (2011) ReaxFF-lg: Correction of the Reaxff Reactive Force Field for London Dispersion, with Applications to Equation of State for Energetic Materials. J Phys Chem 115(40): 11016-11022.
- Gangal SV Grot W (1989) In: Mark H F (Ed.,) Encycl Polym Sci Eng 16: pp 577–648 Wiley, New York.
- Furman D, Kosloff R, Dubnikova F, Zybin SV, Goddard WA et al. (2014) Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms. J Am Chem Soc 136 (11): 4192−4200.
- Tarver Craig M, Tri D Tran, Richard E Whipple (2003) Thermal Decomposition of Pentaerythritol Tetranitrate. Propellants Explosives Pyrotechnics 28(4): p 189-193.
- Cook M (2019) Escondido: Authorities to Burn “Bomb Factory” House.
- Furman D, Kosloff R, Zeiri Y (2016) Effects of Nanoscale Heterogeneities on the Reactivity of Shocked Erythritol Tetranitrate. J Phys Chem 120: 28886.
- Abadie MJM, Sillion B (1991) Polyimides and Other High-Temperature Polymers, Elsevier, Amsterdam.
- Lewis Pat (2004) “Results of PETN PSD Analysis”, personal communication, Lawrence Livermore National Laboratory, Livermore, CA.
- Ghosh MK, Mittal KL (1996) Polyimides. Fundamentals and Applications, Dekker, New York.
- Bykov SV, Mao M, Gares KL, Asher SA (2015) Compact Solid-State 213 Nm Laser Enables Standoff Deep Ultraviolet Raman Spectrometer: Measurements of Nitrate Photochemistry. Appl Spectrosc 69(8): 895−901.
- Emmons ED, Tripathi A, Guicheteau JA, Fountain AW, Christesen SD (2013) Ultraviolet Resonance Raman Spectroscopy of Explosives in Solution and the Solid State. J Phys Chem 117(20): 4158−4166.
- Sharissa Y (2006) Method Development and Validation for Measuring the Particle Size Distribution of Pentaerythritol Tetranitrate (Petn) Powders: Degree of Master of Science in Materials and Metallurgical Engineering, New Mexico Institute of Mining and Technology Socorro, New Mexico.
- Seymour M (1978) Encyclopedia of Explosives and Related Items. PATR 2700, Dover NJ (Eds.,) US Army Armament Research and Development Command, Large Caliber Weapons Systems Laboratory 8: P86-P120.
- Bessonov MI, Koton MM, Kudryatsev VV, Laius LA (1987) Polyimides. Thermally Stable Polymers, Consultants Bureau, New York, pp 318.
- Gares KL, Hufziger KT, Bykov SV, Asher SA (2016) Review of Explosive Detection Methodologies and the Emergence of Standoff Deep Uv resonance raman. J Raman Spectrosc 47: 124−141.
- Yang CP, Hsiao SH (1985) Effects of various factors on the formation of high molecular weight polyamic acid. J Appl Polym Sci 30: 2883.
- Oxley JC, Smith JL, Brady JE, Brown AC (2012) Characterization and Analysis of Tetranitrate Esters. Propellants, Explos, Pyrotech. 37 (1): 24−39.
- Abdelkader H, Hussain Sa, Abdullah N et al. (2018) Review on micro-encapsulation with Chitosan for pharmaceuticals applications. MOJ Curr Res & Rev 1(2):77‒84.
- Hamedi H, Moradi S, Hudson Sm, AE (2018) Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr Polym 445-460.
- Kmiec M, Pighinelli L, Tedesco MF, Silva MM, Reis V (2017) Chitosan – Properties and Application in Dentistry. Adv Tissue Eng Regen Med 2(4): 205–211.
- Nair RS, Morris A, Billa N (2019) An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 20(2): 69.
- Ramaswamy S, Dwarampudi LP, Kadiyala M, Kuppuswamy G, Veera Vendetta Satyanarayana Reddy et al. (2017). Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rut in nanoparticles. Int J Biol Macromol 104(Pt B): 1807-1812.
- Salma M, Abdel-Hafez, Rania M, Hathouth, Omaima A. Sammur (2018) The transdermal penetration pathways of optimized curcumin-loaded chitosan nanoparticles via confocal laser scanning microscopy. Int J Biol Macromol 753-764.
- Silva RFJ, Pighinelli L (2017) Application of Chitosan and Buriti Oil (Mauritia Flexuosa ) in Skin Wound Healing. Journal of Applied Biotechnology & Bioengineering 3(1): 272-279.
-
Pighinelli L, Dias PSR, Paz LR, Guimaraes, FM, Broqua JS, Lisboa CM. Potential Replacement of Pentaeritritol Tetranitrate Explosive Aggregate Polymer (Petn): A Short Review. Arch Biomed Eng & Biotechnol. 2(5): 2019. ABEB.MS.ID.000549.
-
Pentaeritritol, Tetranitrate, Aggregate Polymer, Biological properties, Synthetic polymers, Pentaerythritol, Tetranitrate, polyol, Nitric acid, Pentaerythritol, Erythritol
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






