 Research Article
Research Article
Development and Characterization of Cowry Shell- Based Hydroxyapatite for Dental and Orthopaedic Applications
Abere DV1*, Oyatogun GM2, Oluwasegun KM2, Ojo SA3, Akinwole IE2, Oyatogun AO2, Alabi OO4, Asuquo LO1and Yaskuma U1
11National Metallurgical Development Centre (NMDC) Jos, Nigeria
22Department of Materials Science and Engineering, Obafemi Awolowo University Ile-Ife, Nigeria
33Department of Mechanical Engineering, University of Akron, USA
44Department of Materials and Metallurgical Engineering, Federal University of Technology, Nigeria
DV Abere, National Metallurgical Development Centre (NMDC) Jos, Nigeria.
Received Date:August 10, 2019; Published Date:September 09, 2019
Annotation
This work investigated the suitability of the utilization of cowry shell-based hydroxyapatite (HA) in orthopaedic and dental applications. HA was synthesized via aqueous precipitation process and sintered at different temperatures. The pH and density of the synthetic HA were determined before subjecting the samples to mechanical characterization. The chemical analysis of the HA was carried out with the aid of Energy Dispersive X-ray Florescence (ED-XRF), Atomic Absorption Spectrophotometer (AAS), Fourier’s Transform Infrared Spectroscopy (FTIR) and X-ray Diffraction (XRD) while the microstructural analysis was evaluated using Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS).
The weight of the precipitate produced at pH 9 and 10 are similar to the theoretical HA which is 8.17 g per precipitation batch assuming complete transformation of Calcium (Ca) to HA while the weight recovered at the pH 10 to 12 are greater than the theoretical value and this might be due to the presence of adsorbed water layers on the surface of the powder at the corresponding pH. The density of the synthetic HA is in the range of 2.66–3.75 glcm3 which falls within the theoretical density of HA. The HA has the optimum hardness value of 742 HV at 900oC. The compressive strength obtained ranges from 252.20-452.5 MPa while the optimum compressive strength is 452.5 MPa at 1200oC. The tensile strength obtained is in the range of 55.84 to 86.41 MPa. The optimum value being 86.41 MPa was obtained at 1200oC and this falls within the range of the tensile strength of dense HA. The range of the elasticity of the synthetic HA is 30.83–65.05 G Pa and it was observed that the elasticity of the material increases as the sintering temperature increases. The value obtained is higher than the modulus of bone and that of human tooth but falls within the range of value of a dense HA. The fracture toughness obtained ranges from 0.65 – 2.55 MPam1/2. The optimum value of the fracture toughness which is 2.55 MPam1/2 at 1200oC is within the range of the fracture strength of human compact bone. The ED-XRF and AAS reveal that the main component of the synthetic HA powder are calcium and phosphorus. It can be deduced from the FTIR that the synthetic sample is hydroxyapatite. Observation from the XRD patterns shows that the material is a crystalline single phase with large amount of amorphous phase which is good because amorphous components present an improve biodegradable attributes. Pure HA and other phases in minute concentration were observed in the XRD results. The SEM analysis of the HA material shows that the particle size of the material has a high dispersion. It can be observed that the images of the synthesized hydroxyapatite are porous in nature and this porous nature is a good desirable property of material for bone substitute. The EDS technique reveals that the elemental constituent of the synthesized HA was obtained to be Ca 55.25 wt%, P 26.91 wt% and O 17.84wt% which implies high purity of the calcium phosphate produced through the continuous precipitation technique. The particle sizes obtained through the SEM micrographs are within the range of the sizes that can enhance bone regeneration.
This synthetic hydroxyapatite will be compatible with the human physiological environment since biocompatibility is a direct result of their chemical constituents which include ions that are commonly found in the physiological environment. The synthetic HA will therefore find applications in filling of bone defects in orthopaedic surgery, coating of dental implants and metallic prosthesis.
Keywords:Orthopaedic; Dental; Hydroxyapatite; Precipitation; Crystalline; Bone; Biocompatibility
Introduction
Bones which exist as a natural composite possess type 1 collagen with calcium phosphate in the form of hydroxyapatite in human body [1]. Bone performs several roles which include serving as a calcium reservoir, aiding the soft tissues and providing the cells found in the marrow that differentiate into blood cells and the likes. However, its main function is to serve as mechanical support for soft tissues as well as anchoring for the muscles which generate motion [2]. The case of fractures increases greatly with age and accident. Especially, fractures due to age is partly as a result of extraosseous factors like impaired reflex of the old age, reduced proprioceptive efficiency, reduced cushioning by fat, weakened musculature and by osseous factors such as the structural changes in the shape and size of the bone and by deterioration of the condition of the bone material also [3].
Bone graft materials are quickly becoming a vital tool in reconstructive orthopaedic surgery and demonstrate considerable variability in their appearance. Functions of bone graft maaterials and bone healing provide a structural substrate for these processes and serve as a vehicle for direct antibiotic delivery [4]. The most prominent and major material in the teeth and human bones are hydroxyapatite (HA). Due to this, HA with similar characteristics to natural HA are widely developed to repair and function as bone substitutes.
HA is a crucial element needed for bone regeneration. Various forms of HA have been utilized for a long time. The essence of bone regeneration always revolves around the healthy underlying bone or it may be the surroundings that give sufficient strength. HA is widely known for bone regeneration through conduction or by acting as a scaffold for filling of defects from ancient times, but emerging trends of osteo-inductive characteristics of HA are much promising for new bone regeneration [5]. Emerging technology has made the dreams of clinicians to realize the use of HA in different forms for various regenerative purposes both in vivo and in vitro.
Hydroxyapatite is among the bioceramics which represents the most commercially available regenerative graft material. Hydroxyapatite also belongs to the inorganic components of the bone and is closely associated with the bony apatite structure. It is bounded in the organic matrix, so that it exists with other mineral trace elements in the normal bone [6]. Due to the attribute of the HA, it is attracting more relevant in regenerative science as a good substitute potential material next to autograft. HA has been applied as a substitute for bone due to its chemical nature which is similar to the natural bone. The major constituent of bone is 69 wt% mineral phase, 22 wt% organic matrix and 9 wt% water [7]. Bone is the most prominent calcified tissue in mammals [6] and is a ceramic–organic bionanocomposite with a complex structure. The general formula of HA is Ca10(OH)2(PO4)6 which is similar to an inorganic component of bone matrix. As a result of this similarity, rigorous and extensive research is in progress to utilize HA as a substitute for bone. HA is one of the most stable and less-soluble calcium phosphate bio ceramics with Ca/P ratio of 1.67 [7,8]. The pure HA powder is white but the naturally occurring HA can as well possess green, yellow, or brown colorations, comparable to the discolorations of dental fluorosis. In biological systems, HA occurs as a principal inorganic component of normal (bone, teeth, fish enameloid, and some species of shells) and pathological (dental and urinary calculus and stones) calcifications. The mechanical properties of HA depend on crystal size, porosity, sinterability, phase composition, density and the likes. The bending, compressive, and tensile strength values of HA ceramics respectively fall in the range of 38–250, 120–150, and 38–300 MPa [7,8]. Young’s modulus of dense HA ceramics varies from 35 to 120 GPa, depending on the residual porosity and impurities. Weibull’s modulus of dense HA ceramics lies in the range 5–18, characteristic of brittle materials. The Vicker’s hardness of dense HA ceramics is 3–7 GPa. The mechanical properties of HA bioceramics strongly depend on the microstructure and sintering ability; densely sintered bodies with fine grains are tougher and stronger than porous ones with larger grains [9].
HA bio ceramics have been widely used as artificial bone substitutes due to their excellent biological properties, which among others include bioactivity, biocompatibility, bio affinity, osteoconduction [15], osteointegration [14] and osteoinduction [20]. HA constitutes only calcium and phosphate ions and hence no adverse local or systemic toxicity has been reported in any study. When implanted, newly formed bone binds directly to HA through a carbonated calcium-deficient apatite layer at the bone implant interface [10]. HA surface supports osteoblastic cell adhesion, growth, and differentiation, and new bone is deposited by the creeping substitution from the adjacent living bone. HA scaffolds can also function as delivery vehicles for cytokines with a capacity to bind and concentrate bone morphogenetic proteins (BMPs) in vivo [11]. HA have been investigated for its clinical viability in various bone defects [12]. Many researchers have demonstrated a better initial osseointegration and a high short-term success rate [13-15]. HA-coated implants showed varying results of survival [16-20]. Different forms of HA have been derived from different origins for various uses. Bovine HA [21-30] and synthetic HA [31- 34] are major sources of HA grafts. These have shown varying success rates.
Material and Methods
The raw material for this research is cowry shell purchased from Agbado Oja, Agbado, in Ogun State Nigeria. Some of the reagents used include 0.12 M calcium hydrogen phosphate hydrate [Ca(H2PO4)2.H2O] solution, 0.3 M calcium hydroxide [Ca(OH)2] suspension, 0.3 M orthophosphate acid (H3PO4) solution, 1.0 M ammonium hydroxide (NaOH) solution, 0.3 M ammonium phosphate (NH4)2.HPO4 suspension
Pretreatment operation
The cowry shells were washed thoroughly to get rid of sand, dirt and insect larva and rinsed in deionized water prior to oven dried at 110oC for 30 minutes in order to get rid of the moisture content. The shells were pulverized into powder and subjected to sieve analysis using a sieve machine with meshes 1000 to 50 microns. The powder was calcined in a platinum crucible at 1000oC for 3 hours. The calcium oxide (CaO) formed from the calcination was converted to calcium hydroxide; Ca (OH)2) by treating it with the required amount of deionized water boiled for an hour
Synthesis of hydroxyapatite
The synthesis of HA was achieved through an aqueous precipitation process. The 0.12 M calcium hydrogen phosphate hydrate Ca(H2PO4) 2.H2O solution and the 0.3 M Ca (OH)2 suspension was prepared at room temperature while stirring vigorously for 15 minutes. The Ca(H2PO4) 2.H2O was slowly added to the Ca (OH)2 suspension and stirred gently at room temperature for 60 minutes while the pH was monitored with a Jenway pH meter. The mixture was later subjected to magnetic stirrer and aged for 24 hours at room temperature. The supernatant was decanted while the precipitate was filtered off with filter paper and the residue (HA) was washed with acetone and deionized water to separate it from impurity like NH4 + and the pH was also monitored. The product obtained after filtration was dried in an oven at 110 0C for 24 hrs and the result obtained was made into powder
Sample preparation
Various samples were prepared and sintered at 900, 1000, 1100, and 1200oC to investigate some of the mechanical and chemical properties as well as the phases present in the synthetic hydroxyapatite. The mechanical properties investigated include among others: the compressive strength, tensile strength, hardness, fracture toughness, modulus of elasticity. The average values of five (5) samples of the properties investigated was recorded
Measurement of density
The density of the developed HA was evaluated with the aid of Archimedes’ principle [35]. The volume of water displaced was equal to the volume of the body immersed. The weights of the HA were obtained through Ohaus Scout TM Pro Balance SP2001 equipped with a spring balance as shown in figure 1. The HA sample was suspended in air on the spring with the aid of a thin thread and its weight determined as W,1. It was then completely submerged in a beaker of water and the new weight measured and recorded as W,2. Its density was therefore determined from equation 1 (Figure 1).
Density of HA= (Weight in air)/ (Volume of HA)= W1/(W1-W2 ) 1

Characterization techniques and mechanical tests
Vickers micro hardness, fracture toughness, tensile, compression strength tests and modulus were performed to determine the mechanical properties of the samples. The hardness of the samples was measured with a Vickers micro hardness testing system and performed on the sintered samples at 900, 1000, 1100 and 1200oC respectively. A load of 100 kg-f was applied on the sample for 20 seconds during indentation. Five (5) indentations were performed on each sample and the average was taking, recorded and plotted.
The fracture toughness, tensile and the compression tests were carried out using a Universal Testing Machine
Chemical characterization of the developed HA was performed through Fourier transformed infrared spectro- scopy (FT-IR), with a Perkin-Elmer 2000 FT-IR spectro- meter, Energy Dispersive X-Ray Fluorescence (ED-XRF) and Atomic Absorption Spectrophotometer (AAS). To confirm the presence of hydroxyapatite, X-ray diffraction (XRD) analysis was carried out with the aid of an Advanced X-ray diffractometer (DE) with Cu Ka radiation. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) were employed for microstructural characterization of the developed materials.
Result
The results obtained in this research are presented below (Figure 2-9) (Table 1&2) (Figure 10-12).
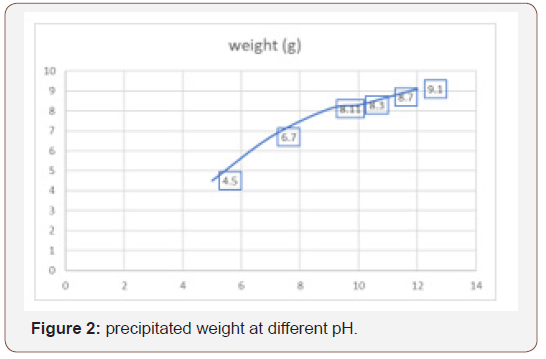
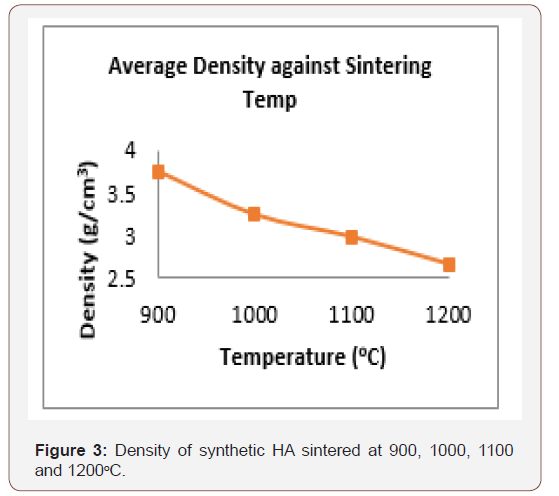
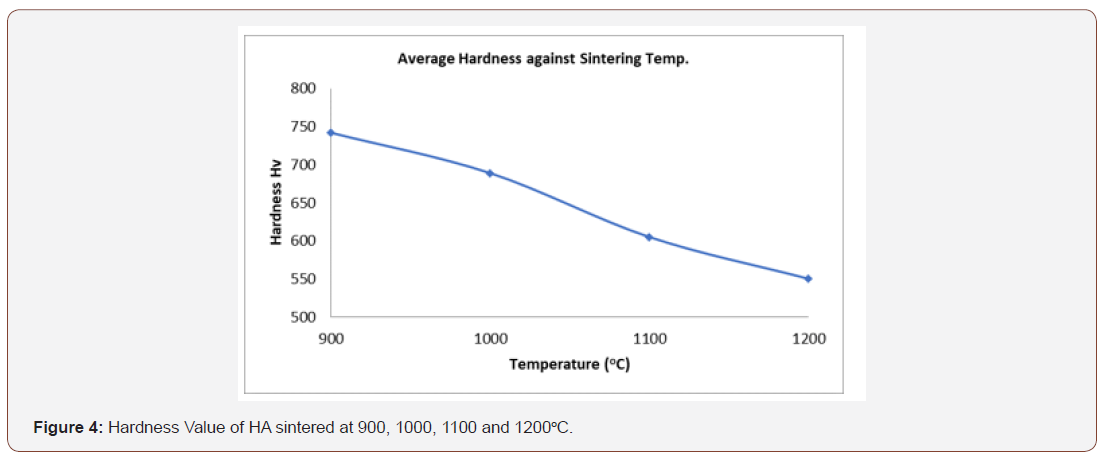
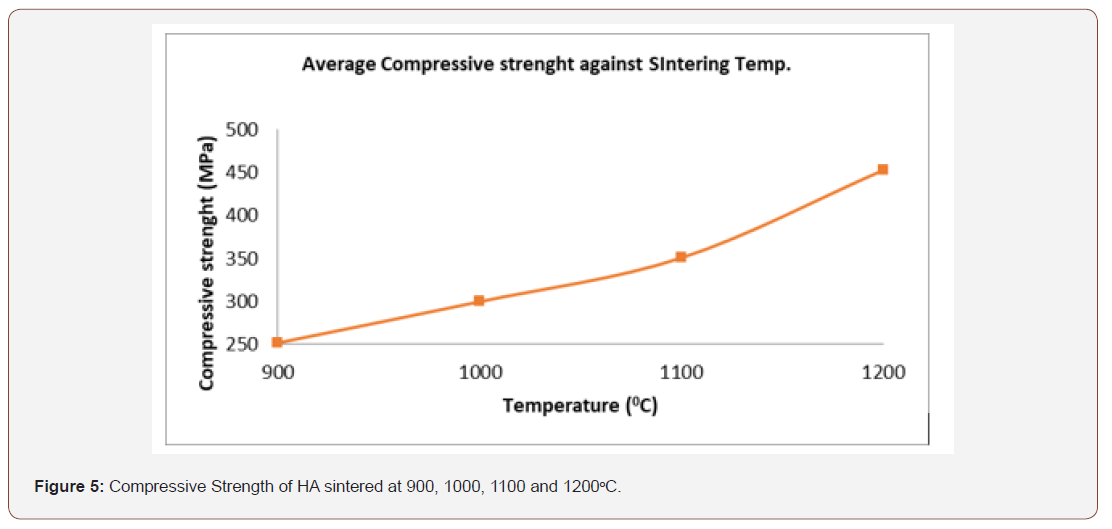
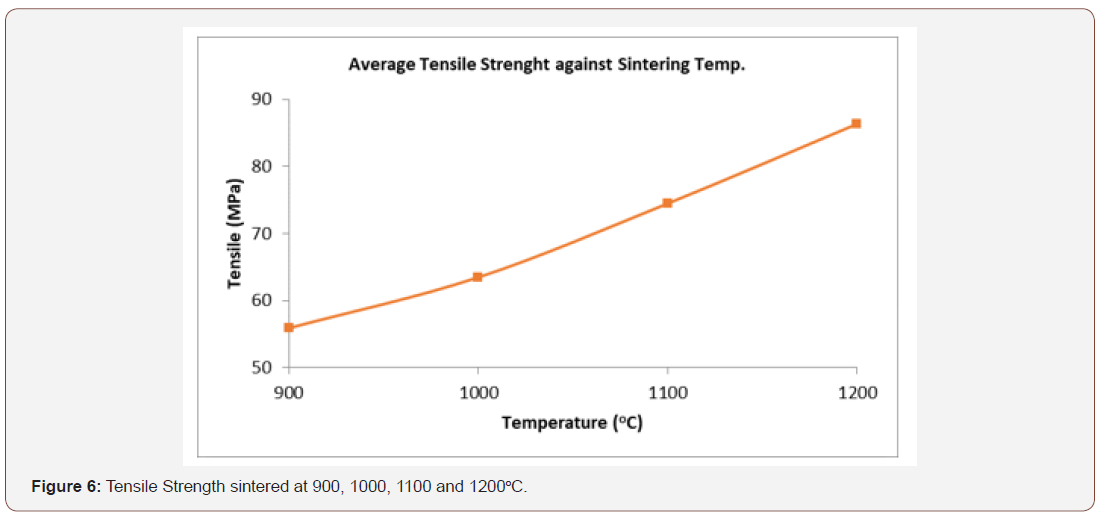
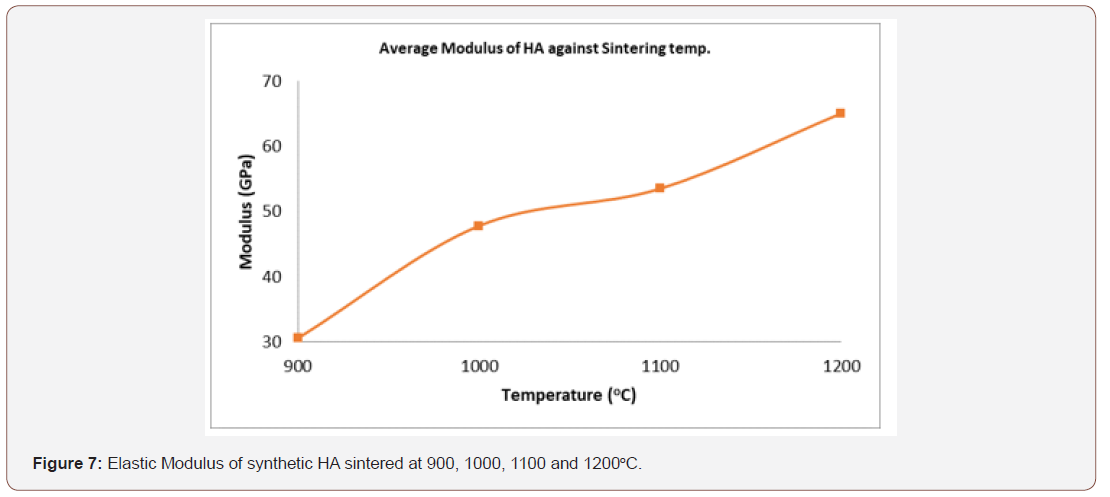
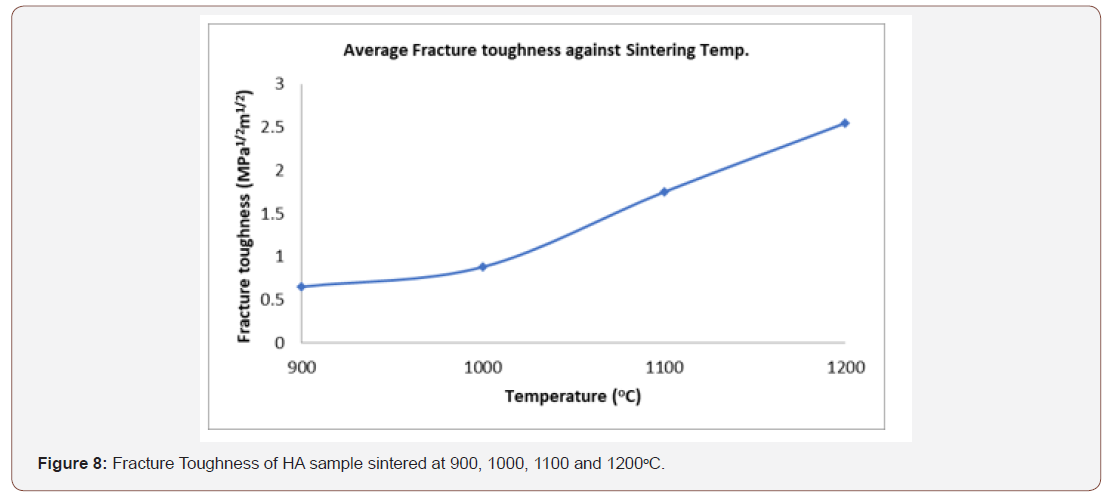
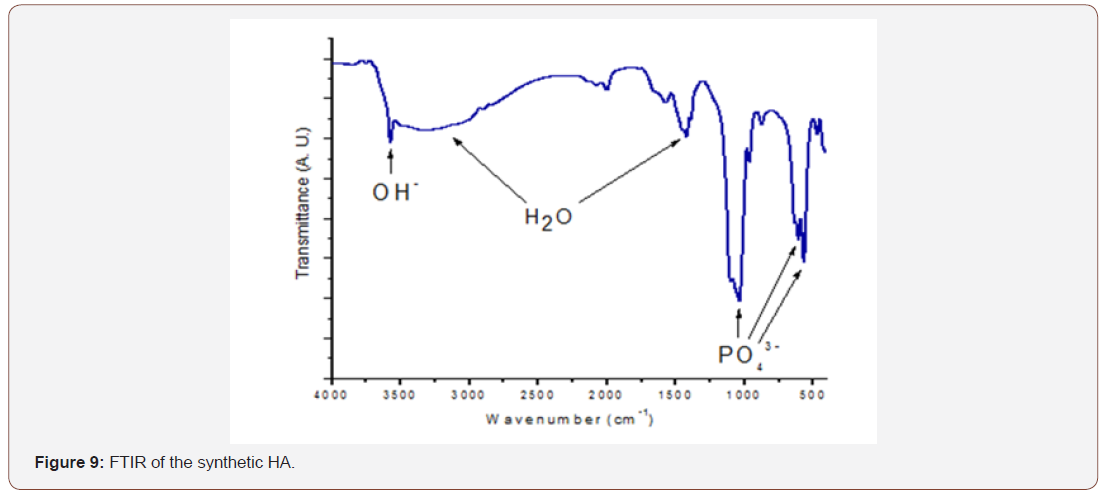
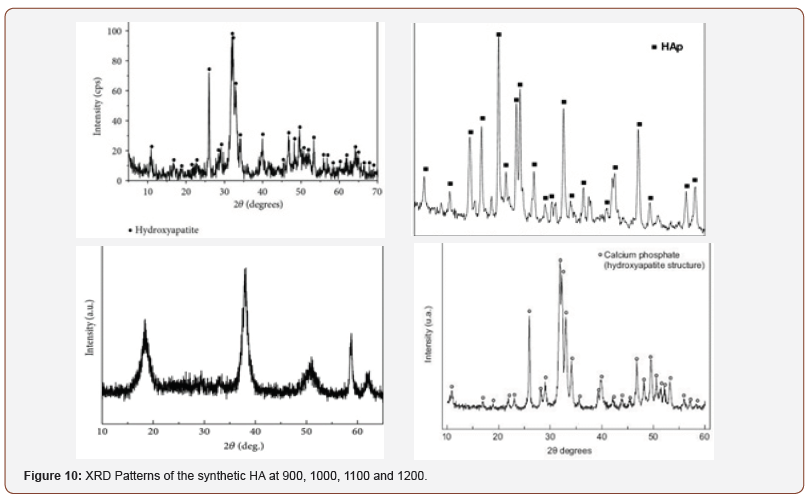

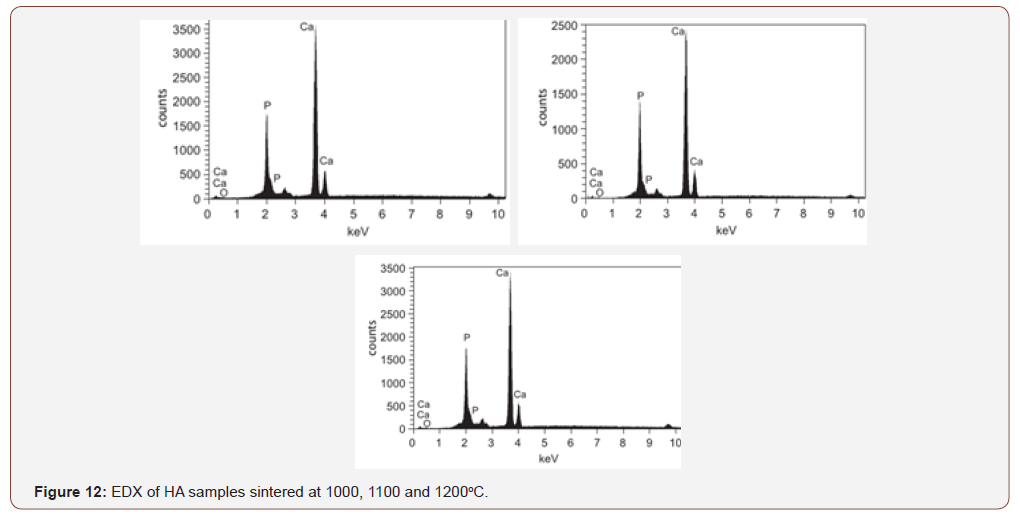
Table 1:ED-XRF Analysis of synthetic HA.

Table 2: Atomic spectrophotometer (AAS) of cowry shell-based HA
Discussion
Effect of pH
The concentration of OH- in the medium of precipitation is among the major prominent parameters in the formation of hydroxyapatite. The pH of the precursor may have effect on the precipitation by changing the solubility of the phases and also affect the agglomeration of the powder. The differences in the solubility of various phases in the precipitation medium can change the structure of the phase and the stoichiometry of the forming phases as the pH increases. The quantity of the precipitates recovered as a function of pH presented in figure 2 reveal that the weight of the precipitate increases as the pH increases i.e., directly proportional. It can be observed also that the weight of the precipitate produced at pH 9 and 10 are similar to the theoretical HA being 8.17 g per precipitation batch assuming complete conversion of Ca to HA. The weight recovered at the pH 10 to 12 are higher than the theoretical value and this might be attributed to the presence of adsorbed water layers on the surface of the powder at the corresponding pH.
Density
Figure 3 presents the density of synthetic HA. The HA with higher porosity and lower density construct provides greater surface area for vascularisation, and bone ingrowth. The optimal conditions for osteo-conductivity are a narrow distribution of inter-connective pores with size in the range 150-300 microns [44]. The density obtained is in the range 2.66 – 3.75 glcm3 but the theoretical density of HA is 3.156 glcm3 [50].
Mechanical and Chemical Properties of Biomaterials
The mechanical properties of biomaterials are very vital in orthopedic and dental implants. Mechanical similarity of the implants to the host or the tissue being replaced is a crucial property. For example, in hard tissue replacements; the biomaterial is required to support or share a portion of the load. Such mechanical properties of biomaterials include tensile strength, compressive strength, fracture toughness, hardness and the likes. The mechanical properties of a particular implant do not depend on the type of metal only but also on the processes used to design the material and device [36].
Hardness
The hardness attribute of a material is very vital and typical requirement when making material selection for medical device applications. In orthopaedic and dental implants for instance, it is recommended that the hardness of the biomaterials be similar to that of the bone being substituted [37]. Kokubo reported that the implants penetrate the bone and cause harm to the host in case the hardness of the implant exceed the bone [38]. From the figure 4, it can be observed that the sintering temperature increase with decrease in the hardness value of the synthetic hydroxyapatite. The HA has the optimum hardness value of 742 HV at 900oC. From [3], the Vickers hardness value of dense HA is 500-800 HV and all the values obtained from the synthetic HA are within this range.
Compressive strength
Hydroxyapatite lacks the mechanical strength needed for long term application in biomedical implants. In practice hydroxyapatite is either used as a bioactive coating on implants or reinforced by metal, polymer, fibre or ceramic phases. The compressive strength obtained from figure 5 ranges from 252.20 - 452.5 MPa while the optimum compressive strength is 452.5 MPa at 1200oC, and these are much higher when compare to the Compressive strength of human compact bone which is 170 – 193 MPa in the direction parallel to the bone axis 133 MPa in the direction normal to the bone axis. Also, the compressive strength of human dentine and enamel are respectively 250-350 MPa and 95-370 MPa and these fall within the limit of the value of the synthetic HA.
Tensile Strength
Strength of implant material has great effect on the fracture of artificial organ. Inadequate strength can cause implant fracture. When the bone implant interface starts to fail, developing a soft fibrous tissue at the interface can make more relative motion between the implant and the bone under loading [45]. This causes the patient to suffer pain and after a while, the pain becomes too much unbearable and hence, the implant requires substitution through a revision surgery [39]. The tensile strength obtained ranges from 55.84 to 86.41 MPa figure 6. The maximum value being 86.41 MPa at 1200oC and this is within the range of the tensile strength of dense HA which is 40-100 MPa [40]. However, the value obtained here could not be used in the human compact bone substitute in the direction parallel to the bone axis to prevent implant failure since the tensile strength of this synthetic HA is less than that of the human compact bone in the parallel axis (124- 174 MPa) but can be applied in the human compact bone at the direction normal to the bone axis [49 MPa]. Whereas, the tensile strength of human tooth (dentine and enamel) are respectively 21-53 MPa and 10 MPa [50] and are within the range of the value obtained, hence the synthetic HA will find applications in filling of bone defects in orthopedic surgery, coating of dental implants and metallic prosthesis.
Modulus of Elasticity
The modulus of elasticity of a material is a crucial property being considered in selecting material for biomedical applications. For major applications such as total joint replacement, must have higher yield strength coupled with the lower modulus similar to that of human bones [39]. The magnitude of the modulus of bone ranges from 4 to 30 GPa depending on the nature of the bone and direction of measurement [17]. If the modulus of the implant is much higher than that of the bone being replaced, it can generate severe stress concentration such as load shielding from natural bone that might make the bone to be weakened and hence, deteriorate the implant/ bone interface, loosening and consequently lead to the implant failure [28,35]. The value of the average modulus of elasticity as shown in figure 7 shows that the range of the elasticity of the synthetic HA is 30.83 – 65.05 GPa and that the elasticity increases as the sintering temperature increases. The value obtained is higher than the modulus of bone and that of human tooth but falls within the range of value of a dense HA [4].
Fracture toughness
The fracture strength is the response of the material to the repeated cyclic loads. Fracture leads to most of the major challenges associated with implant loosening, stress shielding and ultimate implant failure. It is often reported for hip prostheses [34]. The nature of fracture greatly depends on the microstructures of the material. The microstructures of metallic biomaterials change based on the processing and heat treatment technique employed [4]. The value of the fracture toughness obtained as indicated in figure 8 ranges from 0.65 – 2.55 MPam1/2 are within the range of the dense HA. The optimum value which is 2.55 MPam1/2 at 1200oC is within the range of the fracture strength of human compact bone [50].
Chemical analysis
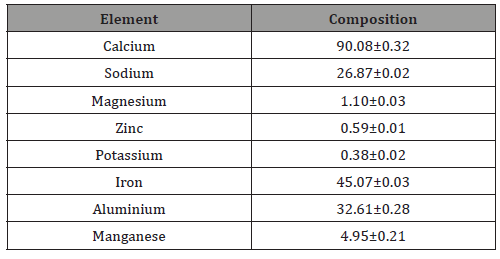
The chemical attribute of a biomedical implant is very vital in functioning of the body. Most ceramics which have been investigated in vivo do not cause increase activity of immune system when dissolved in body fluid or in contact with tissues [16]. However, this synthetic hydroxyapatite will be compatible with the human physiological environment since biocompatibility is a direct result of their chemical constituents which include ions that are commonly found in the physiological environment such as Ca2+, K+,Mg2+, Na+ and of other ions showing very limited toxicity to body tissues such as Al3+, and Ti2+ as shown in table 1.
Calcium is the most abundant mineral present in the perforated cowry shell [2] and this is confirmed in table 2. The high content of calcium confirms its medicinal role in bone formation. It was reported that the cement of the cowry shell can be used as possible cement for bone formation [2] and are used as calcium supplement. Iron, Aluminium and Sodium are found in reasonable amount. Sodium is an extracellular cation involved in the regulation of plasma volume, acid-base balance, nerve and muscle contraction. High dietary sodium has been associated with hypertension. Iron is an important trace element in the human body. It plays prominent roles in haemopoiesis, control of infection and cell mediated immunity. The presence of these minerals contributes to its medicinal value (Sing, 2012). The presence of phytochemicals like Alkaloids, Glycosides, Tannins, Saponins, Quinones in perforated cowry shells, which are biologically important contributes to its value in many areas of medicine e.g. in physiotherapy and pharmacy. Cardenolides/Cardiac glycosides are known to be used in the treatment of congestive heart failure (Schneider and Wolfing, 2004). Saponin inhibits Na+ efflux and activates Na+ and Ca2+ antiporter in Cardiac muscle. The increased influx of Ca2+ which strengthens the contractions of heart muscle and thereby reducing congestive heart failure. Sing reported that cowry marine ecosystems: an environmental protection shells contain some minerals and secondary plant and products which are of biological importance. The reasonable level of sodium in cowry shell is desirable against the backdrop of reported health challenge of high sodium intake. The presence of cardenolides or cardiac glycosides and saponin confirms its therapeutic effects on heart related diseases (Sing, 2012).
From figure 9 the FTIR result revealed that the bands at 565- 645, 960 and 1030 to 1115 cm-1 correspond to phosphate group (PO43-) whereas the band at 3580cm-1 corresponds to OH- group; hence these bands correspond to HA spectrum and are in tandem with [44]. The large gap between the bands reveals the presence of crystalline phase. A weak band of CO₃-² was also detected in the region around 1512.05 cm-1 [41-43]. The wide band at 3041cm- 1 and 1682.40cm-1 were attributed to absorbed water [42]. Hence, it can be deduced that the synthetic sample is certainly hydroxyapatite.
XRD analysis
Figure 10 shows the XRD pattern of the HA powders at 900, 1000, 1100 and 1200oC. Critical observation of the micrographs shows that the material is a crystalline single phase. The analyses of indexation indicate that this material has an hexagonal hydroxyapatite structure with the unit cell parameters determined from the XRD patterns of Ca10(PO4)6(OH)2 (a=b=9.263Å and c=6.941Å) derived from the Suryanarayana et al. process [25]; and this is in tandem with [31]. At the angle 2θ ranging from 20 to 60° the most sharp and intense lines could be observed. These lines correspond with the lines of the XRD spectrum in JCPDS 9–0432 file, which is similar to hydroxyapatite.
SEM analysis
Figure 11 shows the SEM images of hydroxyapatite sintered at 900, 1000, 1100, and 1200oC and it can be observed that the particle size varied from 200 μm to 250 μm and that some particles were agglomerated to the larger size of 250μm to 360 μm showing that the particle size of the material has a high dispersion. It can also be noticed that the images of the synthesized hydroxyapatite is porous in nature and this porous nature is a good desirable property of implant material since it could exhibit positive impact when applied into the dental or orthopaedic implant as it facilitates interaction between the implant and the biological environment [44].
The graphic results of the semi quantitative elemental chemical analysis yielded by EDS technique in figure 12 shows that the elemental constituent of the final synthesized white powders was obtained to be Ca 55.25 wt%, P 26.91 wt% and O 17.84 wt%. These results show high purity of the calcium phosphate produced through the continuous precipitation technique since no other chemical element was detected by the EDS technique when hydroxyapatite was analysed in different zones of the sample
Particle size
From observatiosn, the pore size ranging from 200–400 μm has optimal for cell and bone-tissue regenerations, and enough vascularization [1]. For instance, an in vitro and in vivo study [11] which examined poly(ε-caprolactone) (PCL) scaffolds with various range of pore sizes, revealed chondrocytes and osteoblasts preferred larger pore sizes ranging from 380–405 μm when cultured in vitro. However, when implanted in vivo (cranial defects of rabbits), PCL scaffolds with a lesser pore size of the range 290–310 μm revealed more new bone ingrowth, which moved further into the centre of the scaffold [4]. The particle sizes obtained through the SEM micrographs are within the range of the sizes that can enhance bone regeneration. The pattern of the arrangement of the pores and size of the interconnectivity pose great influence on mechanical properties as well as the osteoconductivity of the implant. The osteoconductive scaffold makes available an appropriate environment for bone cells and bone proteins. The newly placed osteoconductive HA which lacks mechanical bone characteristics gradually acquires mechanical strength which mimic the cancellous bone due to growth of bone after incorporation [45-47].
Conclusion
HA was synthesized via aqueous precipitation process. It was discovered that the HA powder exhibits improved sinter ability and enhanced densification due to a larger surface area, which could improve the mechanical properties of the synthetic HA. The high proportion of calcium as revealed from the ED-XRF and AAS analyses confirm its medicinal role in bone regeneration. The qualitative ED- XRF and AAS reveal that the main component of the synthetic HA powder are calcium and phosphorus. From the XRD analysis with reference to JCPDS number 09-432 indicates the presence of calcium in the material. Through the FTIR analysis the presence of -O-H-, NH⁺⁴ and PO₄³- can be confirmed. From the SEM analysis, it is found that the pores were obtained on the surface of the material and these pores support the osteoblasts to interact and grow over its surface. The EDS technique shows that the elemental composition of the synthesized HA is Ca 55.25 wt%, P 26.91 wt% and O 17.84 wt% which implies high purity of the calcium phosphate produced through the continuous precipitation technique. The particle sizes obtained through the SEM micrographs are within the range of the sizes that can enhance bone regeneration. Hence, by these various characterizations conducted it can be concluded that Hydroxyapatite exists in the prepared sample and thus, will be useful in filling of bone defects in orthopaedic surgery and coating of dental implants [48-50].
Acknowledgement
None.
Conflict of Interest
No Conflicts of Interest.
References
- Hartman GA, Arnold RM, Mills MP, Cochran DL, Mellonig JT (2004) Clinical and histologic evaluation of anorganic bovine bone collagen with or without a collagen barrier. Int J Periodontics Restorative Dent 24(2): 127–135.
- Singh A (2012) Hydroxyapatite a biomaterial: Its chemical synthesis, characterization and study of biocompatibility prepared from shell of garden snail Helix aspersa B Mater Sci 35: 1031-1038.
- Lazic S, Zec S, Miljevic N, Milonjic S (2001) The Effect of Temperature on the Properties of Hydroxyapatite Precipitated from Calcium Hydroxide and Phosphoric Acid, Thermochim. Acta 374(1): 13-22
- Vallet-Regi M, Gonzalez-Calbet JM (2004) Calcium Phosphates as Substitution of Bone Tissues. Progress in Solid State Chemistry 32(1-2): 1-31.
- Ripamonti U, Crooks J, Khoali L, Roden L (2009) The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials 30(7): 1428–1439.
- Hench LL, Thompson I (2010) Twenty-first century challenges for biomaterials. JR Soc Interface 7(suppl 4): S379-S391.
- Dorozhkin SV (2012) Calcium Orthophosphates Applications in Nature, Biology and Medicine. Boca Raton FL: Pan Stanford Publishing.
- Dubok VA (2000) Bioceramics-yesterday, today, tomorrow. Powder Metall Met Ceram 39: 381-394.
- Abere DV, Oyatogun GM, Akinwole IE, Abioye AA, Rominiyi AL, et al. (2017) Effects of Increasing Chitosan Nanofibre Volume Fraction on the Mechanical Property of Hydroxyapatite. American Journal of Materials Science and Engineering 5(1): 6-16.
- Le Geros RZ (2008) Calcium phosphate-based osteoinductive materials. Chem Rev 108(11): 4742–4753.
- Ohgushi H, Dohi Y, Tamai S, Tabata (1993) Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J Biomed Mater Res 27(11): 1401–1407.
- Abere DV, Oyatogun GM, Oluwasegun KM, Ayodele TJ, Ajayi SV et al. (2018) Synthesis and Characterization of Alumina-Chitosan Hydroxyapatite Biocomposites for Load Bearing Application. European Scientific Journal 14(30).
- Ichikawa T, Hirota K, Kanitani H (1996) Rapid bone resorption adjacent to hydroxyapatite-coated implants. J Oral Implantol 22(3-4): 232–235.
- Jones JD, Saigusa M, Van Sickels JE, Tiner BD, Gardner WA (1997) Clinical evaluation of hydroxyapatite-coated titanium plasma-sprayed and titanium plasma-sprayed cylinder dental implants: a preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 84(2): 137–141.
- Simunek A, Vokurkova J, Kopecka D (2002) Evaluation of stability of titanium and hydroxyapatite-coated osseointegrated dental implants: a pilot study. Clin Oral Implants Res 13: 75–79.
- Bifano CA, Edgin WA, Colleton C, Bifano, SL, Constantinom PD (1998) Preliminary evaluation of hydroxyapatite cement as an augmentation device in the edentulous atrophic canine mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 85(5): 512–516.
- Zhou W, Liu Z, Xu S, Hao P, Xu F, et al. (2011) Long-term survivability of hydroxyapatite-coated implants: a meta-analysis. Oral Surg 4: 2–7.
- Artzi Z, Carmeli G, Kozlovsky A (2006) A distinguishable observation between survival and success rate outcome of hydroxyapatite-coated implants in 5–10 years in function. Clin Oral Implants Res 17(1): 85-93.
- Capilla MV, Olid MNR, Gaya MVO, Botella, CR, Romera CZ (2007) Cylindrical dental implants with hydroxyapatite- and titanium plasma spray-coated surfaces: 5-year results. J Oral Implantol 33(2): 59–68.
- Proussaefs P, Lozada J (2004) Immediate loading of hydroxyapatite-coated implants in the maxillary premolar area: three-year results of a pilot study. J Prosthet Dent 91(3): 228–233.
- Callan DP, Rohrer MD (1993) Use of bovine-derived hydroxyapatite in the treatment of edentulous ridge defects: a human clinical and histologic case report. J Periodontal. 64(4): 575–582.
- Hislop WS, Finlay PM, Moos KF (1993) A preliminary study into the uses of an organic bone in oral and maxillofacial surgery. Br J Oral Maxfac Surg 31: 149-153.
- Hämmerle CH, Chiantella GC, Karring T, Lang NP (1998) The effect of a deprotinized bovine bone mineral on bone regeneration around titanium dental implants. Clin Oral Implant Res 9(3): 151–162.
- Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho SC (1998) Sinus floor elevation using anorganic bovine bone matrix (OsteoGraft/N) with and without autogenous bone: a clinical, histologic radiographic, and histomorphometric analysis-Part 2 study of an ongoing prospective study. Int J Periodontal Rest Dent. 18(6): 529–543.
- McAllister BS, Margolin MD, Cogan AG, Buck D, Hollinger JO, et al. (1999) Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int J Oral Maxillofac Implants 14(3): 361–368.
- Kandaswamy D, Ramachandran G, Maheshwari S, Mohan B (2000) Bone regeneration using hydroxyapatite crystals for periapical lesions. Endodontology 12: 51–54.
- Artzi Z, Tal H, Dayan D (2000) Porous bovine bone mineral in healing of human extraction sockets. Part I; histomorphometric evaluations at 9 months. J Periodontol 71(6): 1015–1023.
- Hallman M, Hedin M, Sennerby L, Lundgren S (2002) A prospective 1-year clinical and radiographic study of implants placed after maxillary sinus floor augmentation with bovine Hydroxyapatite and autogenous bone. J Oral Maxillofac Surg 60(3): 277–284.
- Valentini P, Abensur DJ (2003) Maxillary sinus grafting with an organic bovine bone: a clinical report of long-term results. Int J Oral Maxillofac Implants 18(4): 556-560.
- Hallman M, Sennerby L, Zetterqvist L (2005) Lundgren S A 3-year prospective follow-up study of implant-supported fixed prostheses in patients subjected to maxillary sinus floor augmentation with a 80: 20 mixture of deproteinized bovine bone and autogenous bone clinical, Radiographic and resonance frequency analysis. Int J Oral Maxillofac Surg 34: 273–280.
- Bezrukov VM, Gregoriants LA (1998) The surgical treatment of jaw cysts using hydroxyapatite with ultrahigh degree of dispersity. Stomatologiia (Mosk) 77(1): 31–35.
- Chang YL, Lew D, Park JB, Keller JC (1999) Biomechanical and morphometric analysis of hydroxyapatite-coated Implants with varying crystallinity. J Oral Maxillofac Surg 57(9): 1096–1108.
- Mangano C, Bartolucci EG, Mazzocco C (2003) A new porous hydroxyapatite for promotion of bone regeneration in maxillary sinus augmentation clinical and histologic study in humans. Int J Oral Maxillofac Implants 18(1): 23-30.
- Rumpel E, Wolf E, Kauschke E (2006) The biodegradation of hydroxyapatite bone graft substitutes in vivo. Folio Morphol (Warsz). 65(1): 43–48.
- Smith WF, Hashemi J (2006) Foundations of Materials Science and Engineering. 4th Edition, McGraw-Hill, Indiana University, New York, USA
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials Science, An Introduction to Materials in Medicine, 2nd edition, Elsevier Academic Press, San Diego p.162
- Silva VV, Lameiras FS, Domingues RZ (2000) Microstructural and Mechanical Properies of bioceramics. Journal of Biomedical Materials Research 54: 139-148.
- Kokubo T (2008) Bioceramics and their clinical applications. Bioceramics 13: 227-230,
- Wang M (2003) Developing Bioactive Composite Materials for Tissue Replacement. Biomaterials 24(13): 2133-2151.
- Li H, C Zhou, M Zhu, J Tian, J Rong (2010) Preparation and Characterization of Homogeneous Hydroxyapatite/Chitosan Composite Scaffolds via In-Situ Hydration. Journal of Biomaterials and Nanobiotechnology 1(1): 42-49.
- Mobasherpour I, Soulati Heshajin M, Kazemzadeha A, Zakeri M (2007) Synthesis of Nanocrystalline Hydroxyapatite by Using Precipitation Method, Journal of Alloys and Compounds. In Press 430(1-2): 330-333.
- Shuai C, Cao Y, Gao C, et al. (2015) Hydroxyapatite whisker reinforced 63s glass scaffolds for bone tissue engineering. BioMed Research Int 1-8
- Claudia S, Herdocia L, Simara LL, Stefannie Morales, Tania J Gonzalez-Robles et al. (2015) Evaluation of synthesized nanohydroxyapatite nanocellulose composites as biocompatible scaffolds for applications in bone tissue engineering. J Nanomater 1-9.
- Chang B, Lee C, Hong K, Youn H, Ryu H, et al. (2006) Osteoconduction at Porous Hydroxyapatite with Various Pore Configurations Biomaterials 21(12): 1291-1298
- Kim HM, Sasaki Y, Suzuki S (2000) Mechanical Property of Bioactive ceramics and cancellous bones. Journal of Bioceramics 6: 45-48.
- Laura CR, Avram NI, Georgiana AM, Maria Sonmez, Georgeta Voicu et al. (2015) Tetracycline loaded collagen/hydroxyapatite composite materials for biomedical applications. J Nano mater 15.
- Lew D, Farrell B, Bardach J, Keller J (1997) Repair of craniofacial defects with Hydroxyapatite cement. J Oral Maxillofac Surg 55(12): 1441–1449.
- Murugan R, Ramakrishna S (2005) Development of nanocomposites for bone grafting. Composites Science and Technology 65(15): 2385-2406.
- Sadat-Shojai M, Khorasani MT, Dinpanah-Khoshdargi E, Jamshidi A, (2013) Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta biomaterialia 9(8): 7591-7621.
- Weiner S, Wagner HD The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28: 271-298.
-
Abere DV, Oyatogun GM, Oluwasegun KM, Ojo SA, Akinwole IE, Oyatogun AO, e al. Development and Characterization of Cowry Shell- Based Hydroxyapatite for Dental and Orthopaedic Applications. Arch Biomed Eng & Biotechnol. 2(5): 2019. ABEB.MS.ID.000546.
-
Orthopaedic, Dental, Hydroxyapatite, Precipitation, Crystalline, Bone, Biocompatibility, Calcium reservoir, Human bones, Porosity, Sinterability
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






