 Short Communication
Short Communication
Studies on DNA conformational dynamics in Neurodegenerative Brains
Lakshmi Sowmya Emani1, Jayanth KS Rao2* and Jagannatha Rao KS1
1Department of Biotechnology, KLEF Deemed to be University, Vaddeswaram, Andhra Pradesh, India
2Graduate Medical Student, Medical School, University College of Dublin, Dublin, Ireland
Jagannatha Rao KS, Department of Biotechnology, KLEF Deemed to be University, Vaddeswaram, Guntur- 522302, Andhra Pradesh.
Received Date: November 20, 2023; Published Date: December 07, 2023
Abstract
DNA is a key molecule in controlling biological functions. DNA exists in polymorphic conditions like A, B, Z, C and Psi forms and the structural dynamics shows variations in conformational pattern in polymorphic forms and changes in major and minor grooves leads to alterations in transcription pathways. In the human genome, all these forms are invariably appear indicating that DNA in human genome is not static but dynamic. Our own studies in brain indicated B-Z topological change in DNA in hippocampus region in the Alzheimer’s disease (AD) while in Parkinson disease (PD) the DNA helicity is changed to altered B-DNA and the similar conformational changes are observed in bipolar depression brains. Further, in aging brain the DNA undergoes from B-DNA to B-C-A mixed conformation. The studies also indicated the DNA conformational change in AD and PD is due to the translocation of amyloid Beta peptide (A) and synuclein from cytoplasm to nucleus and binds to chromatin. We proposed a hypothesis that how synuclein/ A translocate into nucleus and alter the conformation change in the DNA and induce the strand breaks leading to cell death. We also hypothesize that DNA helical change may become a drug target in near future in neurodegenerative brain.
Keywords:Alzheimer’s disease; Parkinson Disease; DNA Helix; Stability; Neuronal Cell Death; Conformation; Drug Discovery
Introduction
DNA conformational dynamics play a very significant role in the physiology and pathology of cells. DNA is not static but dynamic in the cell. DNA exists in polymorphic conditions, predominately B-DNA, having small portions of Z, tetraplex, and A-DNA [1,2]. The Z-DNA formed a double helix shape with a zig-zag backbone only with minor groove. But in B-DNA, both major and minor grooves are present. The Z-DNA cannot form nucleosomes while B-DNA forms nucleosomes and nucleosomes are essential for cell functions [3]. The Z-DNA conformation is favored by a high GC content [3]. Also, the methylation of cytosine, and molecules like spermine and spermidine can favor the Z-DNA formation and stabilization of the conformation. The specific DNA sequences may also favor B to Z and vice versa transitions. The Z-DNA formation occurs in promoter regions during transcription of genes. During the transcription, the RNA polymerase movement induces a negative superhelix in the front or upstream and one at the rear superhelix or downstream of the transcription. The upstream negative superhelix favors the formation of Z-DNA, while a possible role of Z-DNA could be initiated by negative superhelix [3-5].
DNA consists of two right-handed threads wound around an axis, forming a double helix of 20°A in diameter. The two strands are antiparallel and generally the polymer shows a periodicity of 3.4°A. In the inner part of the molecule, there are the nitrogenous bases, in planes perpendicular to its axis, and this is the hydrophobic part of the molecule. In, outside the molecule, the sugars and phosphate groups form the hydrophilic part. Further DNA-protein interactions are essential in the physiological processes of the cell. The proteins bind to the inner part of the groove of DNA through specific binding at hydrogen bonds, and non-specific binding through Van Der Waals interactions, and electrostatic interactions are common. Some proteins bind to the major groove of the DNA, while others bind to the minor groove. The DNA is ionized under in vivo conditions and behaves as a polyanion. The double helix described above is the “B” of the DNA, which is the most common form under in vivo, although many other DNA forms presence is reported both in vivo and/or in vitro conditions. The “A” form resembles the DNA-B form but is less hydrated. The DNA structures mentioned below have been found to have certain functional roles and observed in different brain disorders like Alzheimer’s (AD), Parkinson (PD), Bipolar (BP) and Depression (D) [6-10].
DNA conformation in age and related disorders brain
The DNA topological changes have been reported in aging brain, AD, PD, D, BP etc and the significant findings are highlighted below.
Aging Brain
Vasudeva raj, et al. [11] reported new evidenced that Copper (Cu), Iron (Fe) elevation and Zinc (Zn) depletion leading to DNA integrity changes in human brain. The study consists of brains from three groups of humans (Group I: below 40 years), (Group II: between 41-60 years), and (Group III: above 61 years). The study focused on Cu, Fe and Zn levels, single strand and double strand breaks and DNA conformation. The results indicated that Cu and Fe levels are significantly increased, and Zn level is decreased from group I to III in frontal cortex and hippocampus. In correlation with the elevation in Cu and Fe, the number of double strand breaks are elevated significantly in frontal cortical region over hippocampus from Group I to III. Also, the DNA underwent B-DNA conformation to altered B-DNA form from Group I to III. This information is very novel and has biological significance in understanding the aging process at molecular level.
Alzheimer’s disease
Suram, et al. [12] reported new evidence indicating that in the normal brain DNA is in B-DNA conformation in hippocampal region. In moderately affected AD brain hippocampus, DNA is in B-Z intermediate form. In severely affected AD brain, the DNA is predominantly in Z-form in the hippocampus region. This indicates the Z-DNA will be in stable form in AD and may modulate gene expression and metabolic pathways.
Parkinson disease
Hegde, et al. [13] reported that DNA helicity and stability changes in eight regions in PD brain. In control brain regions the DNA is in B-form but in PD brain regions the altered B-DNA is found in midbrain, caudate nucleus/putamen, thalamus, hippocampus etc. The DNA stability interns of single and double strand breaks is increased in midbrain, caudate nucleus/putamen, thalamus etc. But in hippocampus of PD brain, the single/double strand breaks has been significantly increased compared to the control brains.
Bipolar disorders
Mustak, et al. [14] studied the conformation and stability of DNA in the brain regions of bipolar depression (BD) compared to age-matched controls. The DNA conformation studies indicated the presence of B-A or secondary B-DNA conformations in BD patients’ brain regions. In control brain regions, the DNA is in B-form. The levels of Cu and Fe are elevated in BD brain regions while Zn levels are depleted. The levels of single and double strand breaks are high in BD brain regions compared to control. They also interestingly observed that genomic DNA is intact in hippocampus with lower elevation of Cu and Fe and the Zn levels did not show significant changes.
All the above studies indicated that genomic stability in terms of conformation and DNA damage is key point in neuronal survivability and cell death. The above genomic changes play an important role in modulating metabolic pathways in brain cells [15]
A proposed mechanism: Does B-Z intermediate and Z-DNA conformational reversal possible back to B-DNA?
DNA exists in polymorphic condition like A, B, Z, Psi, altered B, and C DNA family. In normal cell, B-DNA with structure major and minor groove exists with sub pockets of supercoiled naked Z-DNA, and B-Z intermediary forms. Further, the studies have also shown that right-handed B-DNA can be transformed into Z-form under high salt concentration. Further, the studies indicated a step wise propagation of B-Z junction leading to B-Z transition with the free energy barrier of 13 kcal/mol in the biological system. The changes in free energy between B to Z-DNA are 0.9 kcal/mol per dinucleotide unit thus favouring B to Z transition [16]. Our next major question is, whether can we reverse Z-DNA and B-Z intermediates back to B-DNA?. Zimmer, et al. [17] indicated the binding of molecules like netropsin and dictamycin-3 to Z -DNA and promotes the reversal of Z form to B form. Also, these compounds could be able to bind to B-Z intermediatory forms and then able to reverse to B-form. The above authors have used poly (dG-dC) for Z-form and poly (dA -dT) for altered B-form. Both netropsin and dictamycin-3 are non-chiral molecules with a planarly in symmetry. This molecule binds to oxygen of pyrimidine or N3 of purine in B-DNA and to phosphate sites but in Z-DNA phosphate groups are very close to each other. The guanosine being in syn conformation, the N3 of purines are not accessible for netropsin binding. Further, Geng, et al. [18] reported that amyloid beta (A) aggregates could be able to convert Z-DNA back to B-DNA. However, A monomer could not favour the conversion of Z to B conformational change. Interestingly, the study also indicated that curcumin could block the A induced Z-B conformational transition and thus opening an argument on the role of A and curcumin in DNA conformational transition. The relevance of the study to AD pathology is debatable as Z-DNA is observed in hippocampus in severely affected AD brain and the mechanism is still not understood clearly.
Our hypothesis on the novel role of alpha-synuclein (-Synuclein) in modulating causing DNA conformational change and Damage (Figure 1)
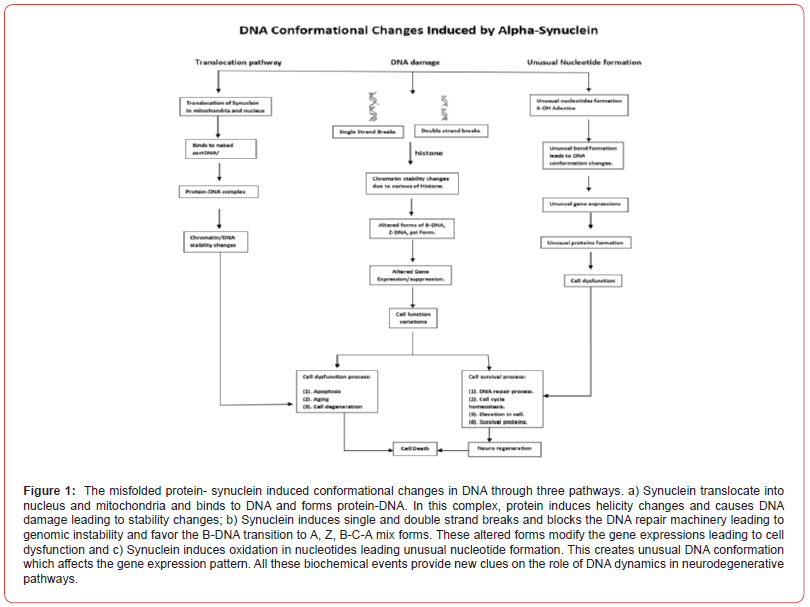
-Synuclein is 140 amino acid protein involved in the pathophysiology of PD. The precise role of alpha-synuclein in normal cell or cell under neurodegeneration as not been completely understood. Hegde, et al. [19] made a first report about synuclein binding to supercoil DNA and induced conformational change from B-form to alter B-form. This data clearly showed DNA binding and modification in DNA integrity by synuclein in clear. Our major question is, whether synuclein translocate into nucleus and binds to chromatin or DNA? . The recent study by Velmarni, et al. [20] clearly showed the nuclear localization of synuclein and evidenced the binding to chromatin and causing damage leading to chromatin destabilization. Also, the higher levels of Fe and Cu support the translocation of Synuclein into the nucleus. In support of this hypothesis, Sangchot, et al. [21] provided novel evidence that the increased Fe supports the translocation of synuclein in human dopaminergic neuroblastoma cell lines. It is very important to understand whether translocated synuclein into nucleus has any functional role? Yan Leng, et al. [22] hypothesized that synuclein may have a role in the regulation PI cycle in the nucleus and linked to phosphatidylinositol related activities in the nucleus.
The DNA damage and the failure of DNA repair plays a key role in early aging and age-related neurodegenerative disorders. Hence, DNA conformational and stability dynamics are considered as one of the important pathogeneses of these disorders and the mechanism of DNA instability is attributed to selective proteins like A, synuclein, TDP43 in neurodegenerative disorders [23- 24]. As indicated above, the translocation of these proteins from cytoplasm into nucleus and mitochondria opened newer understanding on the role of these proteins in DNA conformation and stability. The DNA stability is due to equilibrium between DNA damage and DNA repair. Millanese, et al. [25] showed that the expression of synuclein increases the DNA damage, coupled with the up-regulation DNA damage markers like gamma H2AX, 53BPI and p-ATM in dopaminergic neurons in PD animal model. It is interesting to mention that H2AX normally promotes double strand breaks and the phosphorylated H2AX promotes both double and single strand breaks. In this animal model, synuclein induced both double and single strand DNA breaks as observed in PD brain [13]. The above studies indicated that mitochondrial DNA is very sensitive to damage in dopaminergic neurons andindicated that synuclein translocateinto mitochondria and may imbalance DNA integrity. All the studies demonstrated that DNA damage may trigger proteo-toxicity and also through ROS generation via H2AX phosphorylation. DNA damage response in cell is crucial for promoting cell survivability [24].
Our hypothesis in Figure 1
The studies indicate the role of synuclein in inducing the topological change in DNA conformation and stability. In first pathway, we propose that synuclein translocate into nucleus and mitochondria and then binds to chromatin in nucleus and naked circular DNA in mitochondria leading to formation of protein DNA complex. The above binding changes chromatin/ DNA stability leading to cell dysfunction. In the second pathway, we propose that synuclein induces single/double strand breaks. These strand breaks alter the histone binding pattern and affect the genome integrity. The above modulations not only in DNA conformation but also in DNA repair failure support the genome integrity changes. These changes alter the gene expression thus modulating an imbalance between cell dysfunction/ cell survival. In the third pathway, we hypothesized that the synuclein favours oxidative damage in DNA leading to the formation of unusual nucleotides like 8-H-OH-guanosine, 6 OH adenine, etc. These favour unusual bond formations leads to unusual DNA complexes which promotes the unusual gene expression pattern favouring excess protein pool which leads to cell death.
Conclusion
In conclusion, we believe that DNA stability is maintained in terms of conformation and DNA repair mechanisms in cells. Any imbalance in DNA damage to DNA repair failure leads to genomic instability which affects cell function or favors cell dysfunction. In neurodegenerative diseases, the progressive cell death is critical, and many metabolic pathways get altered hence the identification of drug targets becomes very crucial. So far, the focus is centered on the prevention of A or synuclein aggregation etc. But the therapeutic interventions never become successful. Keeping the above points in view, DNA based drugs seems to be a big hope to retain genomic stability thus control the cell death events in brain.
Acknowledgement
LSE is thankful to KELF Deemed to be University for financial support through fellowship.
Conflicts of Interest
All the authors declare that they have no conflict of interest with reference financial or other interests.
References
- Rasmi Y, Shokati A, Hassan A, Aziz SG, Bastani S, et al. (2022) The role of DNA methylation in progression of neurological disorders and neurodegenerative diseases as well as the prospect of using DNA methylation inhibitors as therapeutic agents for such disorders. IBRO Neurosci Rep 14: 28-37.
- Dileep V, Tsai LH (2021) Neuronal enhancers get a break. Neuron 109(11): 1766-1768.
- Qing X, Zhang G, Wang ZQ (2023) DNA damage response in neurodevelopment and neuro-maintenance. FEBS J 290(13): 3300-3310.
- Fodder K, de Silva R, Warner TT, Bettencourt C (2023) The contribution of DNA methylation to the (dys)function of oligodendroglia in neurodegeneration. Acta Neuropathol Commun 11(1): 106.
- Aboul-ela, F, Bowater RP, Lilley DM (1992) Competing B-Z and helix-coil conformational transitions in supercoiled plasmid DNA. J Biol Chem 267(3): 1776-1785.
- Howell ML, Schroth GP, Ho PS (1996) Sequence-dependent effects of spermine on the thermodynamics of the B-DNA to Z-DNA transition. Biochemistry 35(48): 15373-15382.
- Champion CS, Kumar D, Rajan MT, Rao KSJ, Viswamitra MA (1998) Interaction of Co, Mn, Mg and Al with d(GCCCATGGC) and d(CCGGGCCCGG): a spectroscopic study. Cell Mol Life Sci 54: 488-496.
- Vasudavaraju P, Bharathi, Garruto RM, Kumar S, Rao KSJ (2008) Role of DNA dynamics in Alzheimer’s disease. Brain Res Review 58(1): 136-148.
- Vasquez V, Mitra J, Wang H, Hegde PM, Rao KSJ, et al. (2020) A multi-faceted genotoxic network of alpha-synuclein in the nucleus and mitochondria of dopaminergic neurons in Parkinson’s disease: Emerging concepts and challenges. Prog Neurobiol 185: 101729.
- Vasquez V, Mitra J, Hegde PM, Arvind P, Shiladitya S, et al. (2017) Chromatin-bound oxidized α-Synuclein causes strand breaks in neuronal genomes in in vitro models of Parkinson’s disease. J Alzheimer’s Dis 60: S133-S150.
- Vasudevaraju P, Bharathi, Jyothsna T, Shamasundar NM, Rao KS, et al. (2010) New evidence on iron, copper accumulation and zinc depletion and its correlation with DNA integrity in aging human brain regions. Ind J Psychiatry52 (2): 140-144.
- Anitha S, Rao KSJ, Latha KS, Viswamitra MA (2002) First evidence to show the topological change of DNA from B-DNA to Z-DNA conformation in the hippocampus of Alzheimer's brain. Neuromolecular Med 2(3): 287-295.
- Hegde ML, Gupta VB, Anitha M, Harikrishna T, Shankar SK, et al. (2006) Studies on Genomic DNA topology and stability in brain regions of Parkinson’s disease. Arch Biochem Biophys 449: 143-156.
- Mustak MS, Hegde ML, Dinesh A, Britton GB, Berrocal R, et al. (2010) Evidence of altered DNA integrity in the brain regions of suicidal victims of Bipolar Depression. Indian J Psychiatry 52(3): 220-228.
- Madabhushi R, Pan L, Tsai L-H (2014) DNA damage and its links to neurodegeneration. Neuron 83: 266-282.
- Lee J, Kim YG, Kim KK, Seok C (2010) Transition between B-DNA and Z-DNA: Free energy Landscape for the B-Z junction propagation. J Phys Chem B 114: 9872-9881.
- Zimmer C, Marck C, Guschlbauer W (1983) Z-DNA and other non-B-DNA structures are reversed to B-DNA by interaction with netropsin. FEBS Lett 154(1): 156-160.
- Geng J, Zhao C, Ren J, Qu X (2010) Alzheimer’s disease amyloid beta converting left-handed Z-DNA back to right- handed B-form. Chem Commun. 46(38): 7187-7189.
- Hegde ML, Rao KSJ (2003) Challenges and complexities of alpha-synuclein toxicity: New postulates in unfolding the mystery associated with Parkinson’s disease. Arch Biochem Biophys 418: 169-178.
- Vasquez V, Joy M, Pavana MH, Arvind P, Shiladitya S, et al. (2017) Chromatin-bound oxidized α-Synuclein causes strand breaks in neuronal genomes in invitro models of Parkinson’s disease. J Alzheimer’s Dis 60 (S1): S133-S150.
- Sangchot P, Sharama S, Chetsawang B, Porter J, Govitrapong P, et al. (2002) Transferrin, Ferritin, and Iron in the central and peripheral nervous system. Dev Neurosci 24: 143-153.
- Leng Y, Chase TN, Bennet MC (2001) Muscarinic receptor stimulation induces translocation of an alpha-synuclein oligomer from plasma membrane to a light vesicle fraction in cytoplasm. J Biol Chem 276(30): 28212-28218.
- Anitha S, Hegde ML, Rao KSJ (2007) A new evidence for DNA nicking property of amyloid beta-peptide (1-42): relevance to Alzheimer's disease. Arch Biochem Biophys 463(2): 245-252.
- Mitra J, Erika NG, Hegde PM, Nicole FL, Wang H, et al. (2019) Motor Neuron Disease-Associated Loss of Nuclear TDP-43 is Linked to DNA Double-Strand Break Repair Defects. Proc Natl Acad Sci U S A 116(10): 4696-4705.
- Milanese C, Cerri S, Ulusoy A, Simona V, Gomati P, et al. (2018) Activation of the DNA damage response in vivo in synucleinopathy models of Parkinson’s disease. Cell Death Dis 9(8): 818-830.
-
Lakshmi Sowmya Emani, Jayanth KS Rao* and Jagannatha Rao KS. Studies on DNA conformational dynamics in Neurodegenerative Brains. Arch Biomed Eng & Biotechnol. 7(4): 2023. ABEB.MS.ID.000669.
-
Alzheimer’s disease; Parkinson Disease; DNA Helix; Stability; Neuronal Cell Death; Conformation; Drug Discovery
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






