 Research Article
Research Article
Development of a New Tehnique in Cytology, Protocol for Direct Staining in the Test Tube of Cervico-Vaginal and Samples
Ana Maria Cretu1,2*, Nicolau Anca Antonela1,2, Liliana Seicaru2, Miruna Cristian1,3 and Tase Geta Manuela2
1Centre for Research and Development of the Morphological and Genetic Studies of Malignant Pathology (CEDMOG), “Ovidius” University, 900591 Constanta,Romania
2Department of Pathology, Clinical Service of Pathology, “Sf. Apostol Andrei” Emergency County Hospital, 900591 Constanta, Romania
13Department of Forensic Medicine, “Sf. Apostol Andrei” Emergency County Hospital, Constanţa, Romania
Ana Maria Cretu, Centre for Research and Development of the Morphological and Genetic Studies of Malignant Pathology, (CEDMOG), “Ovidius” University, 900591 Constanta, Romania
Received Date:March 19, 2025; Published Date:March 27, 2025
Abstract
Introduction: Papanicolaou smear is a widely used method for diagnosing precancerous and cancerous lesions, estimating hormonal status
and the presence of possible infections, and is the only method used in cervical cancer screening programs. Since the number of samples to be
evaluated in these national programs is very large, it is necessary to reduce as much as possible the time allocated in processing the smears that will be evaluated. For that, we tested a quick, modified Papanicolaou staining method (Paper).
Materials and methods: Qualitative and quantitative experimental research was carried out on 70 biological products harvested on
liquid medium, respectively cervical-vaginal cell samples. The samples were divided into 2 groups. Group A: Papanicolaou stain (PAP), applied
on conventional smears and group B: modified Papanicolaou stain (Paper), applied directly to sediments obtained after centrifugation, in a test
tube, without prior display on slides. The final smears were obtained by the method of cytology in liquid medium, in monolayer (liquid-based
cytology, LBC). The two types of approaches were compared qualitatively and quantitatively. Also, the two methods were analysed by comparing
the final execution times. The quality of staining was initially classified as optimal, good and poor, with subsequent reclassification to adequate and inadequate for evaluation. The data was entered into Microsoft Excel and further statistical analysis was performed using the software IBM SPSS
Statistical Analysis Software Release 30.0.0. Cytological interpretation was performed according to TBS 2014 recommendations.
Results: All cellular details described by TBS were noted in the Paper method compared to PAP (p < 0,001). As a percentage, the average of
the remaining sediment, ready to be used in the work procedure, it was 70%. Conclusions. Staining directly in the test tube is a new, effective and
innovative method that reduces the use of reagents and the time allocated to the procedure, generating performance in the diagnosis of cervical
cancer. Thus, in just 3 minutes, a macroscopically stained sample can be obtained, from which multiple smears can be performed, the loss of
cellularity being relatively small, the artifacts that appeared due to delayed fixation are eliminated. A smaller amount of dye solutions and fixing
solutions is also used and contamination of cell solutions avoids contamination from nearby biological products. The disadvantages of the method
would be the total exhaustion of the harvested material and its limitation only to cytopathological reassessment, useful for screening.
Keywords:Papanicolaou Stain; Cervical-vaginal Cytology; Cervical Cancer Screening
Introduction
Papanicolaou smear is a method that uses polychrome dye, and is widely used for diagnosing precancerous and cancerous lesions, estimating hormonal status, and status of cervical infections [1,2]. Papanicolaou smear is relatively economical and effective, which has allowed the detection of cervical cancer in high-income countries, dramatically lowering the mortality rate and is still the basis of the screening protocol, in cervical cancer screening and prevention programs worldwide [3-5]. In the cytopathology laboratory, cytological smears are usually performed on several slides, which are subsequently fixed in specific fixative solutions and then stained by successive immersion in specific dyes, water baths and dehydration solutions. During the staining process that lasts about 36 minutes, until conventional smears are obtained, a number of artifacts can also appear, due to faulty techniques, such as: delayed processing can lead to degeneration of the smear image with loss of cell morphology and a lot of bacteria in the background smear; delayed fixation can lead to air-drying artifacts (pale nuclei, lack of differential cytoplasmic staining, cytoplasmic and nuclear eosinophilia); contamination from other smears and cells from smears to spill onto other slides (all staining and fixing solutions used should be filtered daily using filter paper) [6-15].
Unfortunately, many laboratories are faced with the problem of the time allocated to processing the slides before they are microscopically evaluated, since the Papanicolaou smear technique involves allocating a relatively high time of performance, through the times allocated to each specific dye, or washing, moisturizing and dehydrating with alcohols of different concentrations, the use of glassware and other consumables that all add up. This highlights the need to look for faster, simpler and cheaper alternatives for diagnosing cervical cancer through exfoliative cytology.
From the 1950s to the present, changes in conventional Papanicolaou staining have been developed in its various components, stages and staining times. Haematoxylin, a nuclear dye, underwent substantial modifications on the same chemical basis, preventing the use of other plant derivatives for this purpose [16-18]. Thus, we set out to validate the use of a much faster, testtube staining that would comply with the same high performance required for evaluation and interpretation by staining cell nuclei and cytoplasmic components without errors. This is a challenge for research in laboratories where the volume of biological products to be analysed is high, requiring innovation techniques that benefit public health. We will also include the ready-stained sediment in the cytoproct, according to the method approached by us and described in a previous work [19].
Material and Methods
Experimental research was performed at the Centre for Research and Development of the Morphological and Genetic Studies of Malignant Pathology (CEDMOG), during 2024. The samples were derived from external clinics or from health networks and micro networks transmitted by agreement with the institution. The study included all cervical smears that met the sampling quality criteria described in the institution’s Manual of Technical Competence and Standard Procedures, the Manual of Standard Operating Procedures, and also included the pre-analytical considerations required by the Bethesda System (TBS) 2014. Data collection and sample processing are described below.
Pre-analytical stage
Qualitative and quantitative experimental research was carried out on 70 biological products obtained by exfoliation, represented by cervical-vaginal secretions. The samples were collected on a liquid medium, in healthcare centres, with an Ayre spatula and yoctometre brush (cervix brush) by obstetricians. All smears were immediately fixed in alcohol-based fixing solution (Sure Path, 10ml) and sent to the Cytology laboratory, where they were received, coded and pre-processed. The entry code of each sample was recorded in the register of case records.
Analytical stage
The samples were processed by 2 different staining methods, the first being a standardized method, currently used in current practice in cytology laboratories, and the next one method underwent changes in pre- staining processing, but also with strategies to eliminate and reduce reagents and execution times of the 70 samples, 3 were removed from the study due to paucicellularity, measured with the Cyto-Fast System for vaginalis liquids sampling, with 67 samples remaining in the study, considered compliant for evaluation. Also, for the application of specific stains, directly in the test tube or on smears, it was necessary to prepare the sediment consisting of the cells of interest beforehand. The selection was made based on the analysis in terms of cell density, so that the analysed samples were divided into 4 groups: a. hypocellular, b.cu optimal cellularity, c. weak cellular samples and d. haemorrhagic samples. The 4 types of products were balanced in terms of cell density, either by reducing cellularity, by adding additional fixative solution, or by lysis of red blood cells in haemorrhagic samples. For haemorrhagic products, glacial acetic acid 100% (Merck, Darmstadt, Germany) was used to determine targeted cytolysis of red blood cells, without altering inflammatory cells, epithelial cells or vaginal flora.
Subsequently, all the samples remaining in the study were centrifuged at 2000 rpm for 10 minutes with the Hettich 32A liquid media centrifuge. The entire sediment was divided into three equal parts, and noted as follows: group A and B. Group A, was subjected to Pap smear, applied on conventional smears, spread and fixed in advance. Group B involved staining the cell sediment directly in the test tube, based on the dyes from the Papanicolaou kit (Merck, lot 2024, Darmstadt, Germany), with reduced exposure times to alcohol. The times allocated differ very little from those noted in the classic staining technique, on smears, but they were adapted according to the dyes used.
For group A (PAP), the basic procedure for Papanicolaou smear staining was applied, which includes
12 immersion steps, which take place, in total, in about 50 minutes. This measured time also included the initial centrifugation of the biological product, to obtain the study sediment. The stages of the staining technique were: fixing the smear with 95% ethanol for 15 minutes; rinsing in tap water, 1 minute; adding Harris Haematoxylin dye for 3-4 minutes; it is then differentiated into 0.5% aqueous hydrochloric acid for approximately 10 seconds, after which it is rinsed in tap water, 2 minutes; immersion of the preparation in 95% ethanol 10 dips, 2 minutes; then immersed in OG-6 (the first acidic counterstain, is a cytoplasmic stain, which stains matured and keratinized cells, the target structures are stained orange in different intensities), dye solution for 1.5 minutes; ethanol 95% 10 dips, 2 minutes; eosin dye bath - EA-50 (Eosin Azure : the second counterstain, a polychrome mixture of eosin, light green and Bismarck brown) for 3 minutes; immersion in ethanol 95% 10 dips, 2 baths, 2 minutes; immersion in 100% ethanol for 1 minute; clarification in 2 xylene baths, 2 minutes each; then the slat is mounted, with permanent mounting medium for 2 minutes.
For group B (Paper), the steps of the test-tube staining procedure, with Papanicolaou smear, were: centrifugation of the sample at 1000 rpm/5 minutes; dehydration stage with 70% ethyl alcohol, immediately followed by centrifugation 1000 rpm/5 minutes; addition of 2ml of Harris Haematoxylin dye for 2 minutes, followed by a dye removal step and at the same time rehydration with 10 ml 50% ethanol, for 1 minute; spin1000 rpm/5 minutes; addition of a mixture of OG- 6 and EA 50 dyes, in a ratio of 1:2, for 3 minutes; wash 2 times with 5 ml 50% ethanol and spin 1000 rpm/5 minutes. After the final centrifugation and removal of the supernatant, approximately 0.2 ml of sediment is obtained, macroscopically visibly stained, sufficient for performing several cytological smears. For the qualitative evaluation of the coloration, it was only necessary to display 2 smears of each sediment, the rest was kept in the refrigerator, at 4oC, for subsequent smears. The smears thus obtained were air-dried and permanently mounted with mounting medium. Each reading was taken on 2 smears, the number of intact cells attached to each glass slide being counted using a Zeiss Primo Star optical microscope, with 10x, 20x and 40x objectives, respectively. Cell counting was performed as a whole, on each coloured specimen, and the background was evaluated for its colour, and the edges of the coloured pill were examined for colour and integrity.
Post-analytical stage
The results were recorded in the service report register, after validation by the laboratory staff (cytotechnologists and cytopathologists). Subsequently, they were delivered to patients (through the coordinators of the health networks) within the stipulated deadlines.
Statistical analysis
The data was entered into Microsoft Excel and further statistical analysis was performed using the software IBM SPSS Statistical Analysis Software Release 30.0.0.
All diagnostic test parameters were used to assess the diagnostic concordance between conventional staining and the PapR method. Thus, for the calculation of these parameters, the optimal certainty test and the data obtained from the application of the standard PAP smear were used as a comparison protocol. In order to numerically quantify the cytological qualitative assessment, the parameters used in the statistical calculations were obtained by the prior calculation of the degree of suitability for interpretation (DSI)..
Results
After the first centrifugation of the biological products, an average of 1.5 ml of sediment was obtained. After determining cell density, the balancing was chieved as follows: was suspended the pellet in the preservative solution to obtain an unclear solution. For a. isocellular sample or practically lack of pellet, b. cellular sample, c. very cellular sample and sg. hematic sample (treated in the same way, to obtain a not so dense solution), but not before the lysis of red blood cells is induced (Table 1). What could not be done, however, was the balancing of cell types (the device detects all cell types, either inflammatory cells or squamous cells, without differentiating between them).
Table 1:Initial classification of samples according to cell density.

Qualitative analysis (clarity and intensity of staining, as well as preservation of cellular and nuclear characteristics) was performed using the stain quality index, the Bethesda System, and the quality program for external cytology evaluation. The quality of staining was classified as optimal, good and poor, with subsequent reclassification to adequate and unsuitable for evaluation. Thus, for PAP, the Degree of Suitability for Interpretation (DSI) = 0,86 (with an ideal of 1), and for PapR (DSI) = 0,81.
The cytological evaluation included the evaluation from a morphological and chromatic point of view, based on the ticking of the criteria required to be considered suitable smears for interpretation: aspects of cell morphology, general presentation of staining, aspects of the chromatic pattern, nuclear staining and continuity of the cell and nuclear membrane, including the background of the smear and the presence of intact vaginal flora. In order to numerically quantify the cytological qualitative assessment, the parameters used in the statistical calculations were obtained by the prior calculation of the degree of suitability for interpretation (DSI) (Table 2).
Table 2:Qualitative cytological evaluation.

Morphological integrity refers to keeping cell membranes intact in the absence of cytolysis. The cell morphology was assessed according to the degree of privatisation of the cell cytoplasm’s and the arrangement of the cells in overlapping piles. In order to assess the general presentation of the stains, the presence of the three basic colours highlighted by the conventional PAP staining was used as a reference criterion, namely: purple (for nuclei and nucleoli), orange (for keratinization and keratohyalin drops) and blue-green (cytoplasm of metaplastic cells). In the Paper, the presence of all three colours was noted. The chromatic model involved noting the presence of cytoplasmic amphophilic and polychromatophilia specific to vaginal secretions associated with specific or non-specific inflammation or infection, as well as the presence of intracytoplasmic glycogen in intermediate squamous cells (Figure 1).
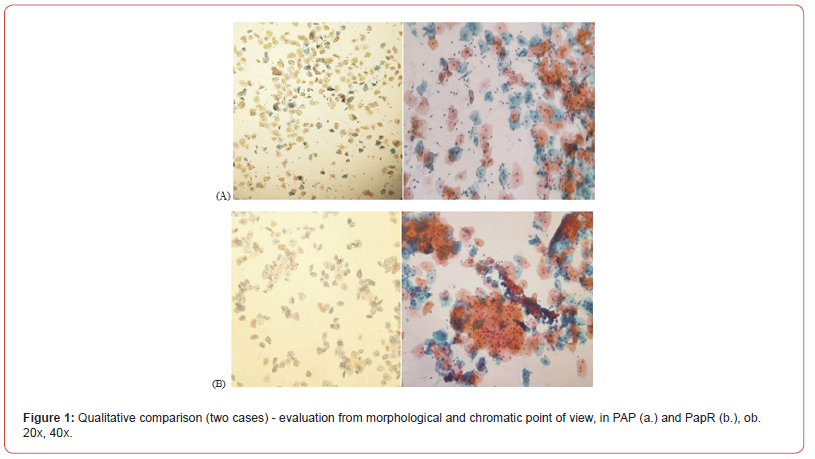
Cytological interpretation was performed according to TBS 2014 recommendations. As for the cytological details, they were identified on both investigated methods. All cellular characteristics (cytological and nuclear) corresponding to the findings on the PAP smears were also found on the Paper smears. Thus, diagnoses of non- neoplastic smears (with normal cytology, NLIM), infectious and/or inflammatory smears (with reactive cytology), smears with squamous epithelial cell abnormalities (ASCUS, ASCH and LSIL) and smears with glandular atypia of undetermined significance (AGCNOS) were noted. There were no cases of squamous cell carcinoma or adenocarcinoma.
The changes occurred in the Paper staining technique or in the smear display stage, did not distort, modify or suppress the cytological details so as to alter the diagnoses previously given and considered to be diagnoses of certainty. This is evidenced by photomicrographs (Figure 2).
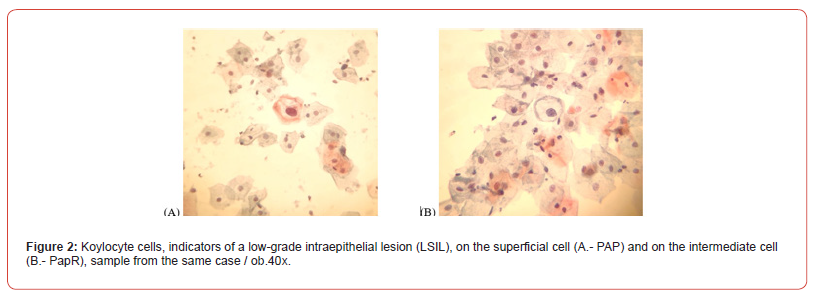
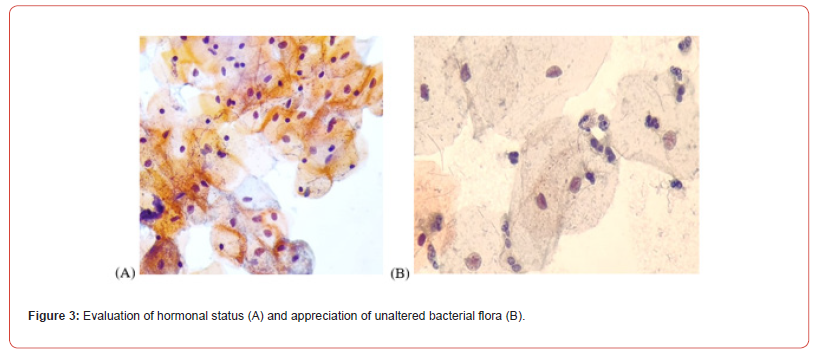
In order to assess the clarity of the cytoplasm, it was taken into account the observance of the colour intensity characteristic of each cell type (superficial squamous cells have eosinophilic cytoplasm, coloured in pale pink, intermediate squamous cells have basophilic cytoplasm, coloured pale blue, basal and parabasal cells have basophilic-slightly capnophilic cytoplasm, intense, metaplastic cells have capnophilic cytoplasm, intense), the presence of keratohyalin droplets and glycogen which demonstrates optimal absorption of dyes. The presence of intracytoplasmic glycogen, keratohyalin drops, and colour intensity in the cytoplasm provide additional information on hormonal status, and the presence of intact vaginal flora is also important in the final diagnosis. In the figure 3, we can note all the three characteristics mentioned.
The assessment of the clarity of the nuclear chromatin was made by noting the presence or absence of nucleoli, and of the optically empty spaces and of the obvious, slightly thickened nuclear contour (nuclear integrity). An opaque nucleus, it was considered “faintly” coloured (Figure 3).
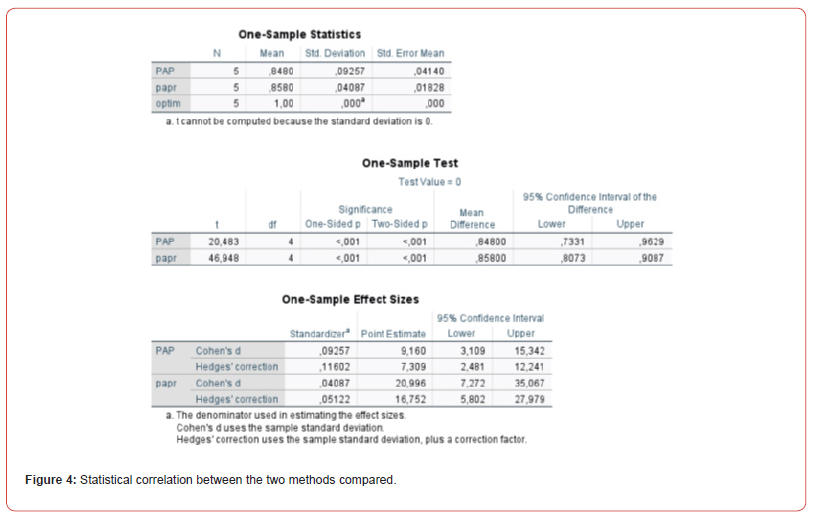
All cellular details described by TBS were noted in the Paper method compared to PAP (p < 0,001). Also, Paper staining had a sensitivity of 85,67 and a specificity of 14,32, a positive predictive value (PPV) of 92, a negative predictive value (NPV) of 81, a false negative rate of 6,56%, and a false positive rate of 2,68%, all data are percentage, calculated with a 95% confidence interval. We also compared the two staining methods by Cohen’s d calculation, which characterizes the effect size by correlating the mean difference with the variability, similar to a signal-to-noise ratio. A large Cohen d indicates that the mean difference is large compared to the variability. Cohen suggested that the d-values of 0.4 represent a small difference between the two methods (Figure 4).
Finally, after finishing the staining in the test tube, the samples were analyzed quantitatively (how much sediment remained after staining in the test tube – if an average of 1,5 ml was used before staining, at the end of staining an average of 1,05 ml remained in group B. As a percentage, the average of the remaining sediment, ready to be used in the work procedure, is 70%. Thus, for 23 samples the remaining sediment was 1,1 ml, for 15 samples/1.3 ml and for 29 samples/0,9 ml. Also, the number of smears that can be spread on the slides depends on the remaining quantity (Figure 5).
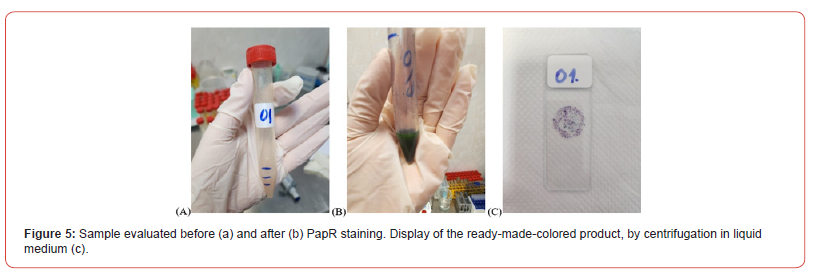
The sample thus obtained, being fixed, has stability of up to 7 days, if it is kept in the refrigerator at 4oC. Samples are stored longer if stored in dark bottles with an airtight cork to prevent evaporation. Haematoxylin retains relatively constant staining characteristics, while the OG 6 and EA 50 reagents lose their strength more quickly, about after 7 days (the cells become pale, poorly stained, although they retain their morphological integrity).
Conclusion
Direct test-tube staining (Paper) is a new, effective and innovative method that reduces the use of reagents and the time allocated to the procedure, generating performance in the diagnosis of cervical cancer. If for the basic staining procedure, by the classical Pap method, approximately 50 minutes is allocated, including the initial centrifugation of the biological product to obtain the study sediment, in the case of staining in the Paper test tube, the procedure time is reduced to 3 minutes (94% less than in the PAP method). The loss of cellularity is relatively small, contributing to this the stages of cell density balancing, which includes the elimination of red blood cells by cell lysis, in the case of haemorrhagic products.
Thus, in 3 minutes a macroscopically coloured sample can be obtained, from which multiple smears can be performed (approximately 10-30 smears, the number varies depending on the amount of product taken, but also depending on the particular cellularity of each product). In this way, rapid, multiple smears can be obtained, which can be accessed as needed and considered satisfactory for interpretation, which provide reliable results on a possible malignancy (by observing cytoplasmic and nuclear characteristics), but also on hormonal status and vaginal flora. Paper staining can be used as an alternative to the conventional Pap smear used in cervical-vaginal exfoliative cytology, as an equally efficient method of processing the biological samples, but much faster, with obtaining multiple and similar smears, thus ensuring faster conditions for reading and interpreting the smears, especially useful in the case of screening that involves a large volume of samples and requires results and diagnoses in a shorter time.
Other advantages of this method would be: elimination of possible artifacts that may occur due to delayed fixation, sealing of solutions in the dye battery and using a large amount of dye and fixation solutions, avoiding contamination with cells from other neighbouring biological products, frequent washing after each use of immersion vats that include significant loss of time. The disadvantages of the method would be the total exhaustion of the harvested material and its limitation only to cytopathological revaluation.
Disclosure of Conflict of Interest: None to declare. Acknowledgement
I would like to express my sincere gratitude to the members of the research team who contributed to the successful completion of this study. Their dedication, expertise and commitment have been essential in achieving our research objectives. I am grateful for their valuable insights, collaborative spirit and unwavering support throughout the project.
References
- Papanicolaou N, Traut HF (1941) The diagnosis value of vaginal smear in carcinoma of the uterus. Am J Obstet Gynecol 42(2): 193-206.
- Papanicolaou G. Atlas of Exfoliative Cytology (2018) Cambridge: Harvard University Press 1954.
- Harjes U (2018) Pap seeking new challenges. Nat Rev Cancer 18(6): 338-339.
- Catarino R, Petignat P, Dongui G, Vassilakos P (2015) Cervical cancer screening in developing
- Arbyn M, Herbert A, Schenck U, P Nieminen, J Patnick, et al. (2007) European guidelines for quality assurance in cervical cancer screening: recommendations for collecting samples for conventional and liquid-based cytology. Cytopathology 189(3): 1333-1339.
- Gupta S, Chachra KL, Bhadola P, Sodhani P (2010) Modified Papanicolaou staining protocol with
- Rajesh K, Chaurasia, Kapil B Shirsath, Balvinder K Sapra (2021) Protocol for one-step selective lysis of red blood cells and platelets with long-term preservation of white blood cells (human) at ambient temperature. STAR Protoc 2(4): 100834-100839.
- Manjiri Milind Makde MD, Prajakta Sathawane (2022) Liquid-based cytology: Technical aspects Cytojournal 19(41): 1-10.
- George N Papanicolaou (1942) A New Procedure for Staining Vaginal Smears Science 24( 95): 438-439.
- Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, et al. (2006) Effect of study design and quality on unsatisfactory rateş, cytology classifications, and accuracy în liquid-based versus conventional cervical cytology: a systematic review. Lancet 14(367): 122-132.
- Guidos BJ, Selvaggi SM (1999) Use of the ThinPrep Pap Test în clinical practice. Diagn
- Jörundsson E, Lumsden JH, Jacobs RM (1999) Rapid staining techniques in cytopathology: A review and comparison of modified protocols for hematoxylin and eosin, Papanicolaou and Romanowsky stains. Vet Clin Pathol 28(3): 100-108.
- Moya-Salazar J, Rojas-Zumaran V (2016) Validation of the modification of the prolonged
- Choudhary P, Sudhamani S, Pandit A, Kiri VM (2012) Comparison of modified ultrafast Papanicolaou stain with the standard rapid Papanicolaou stain in cytology of various organs. J Cytol 29(4): 241-245.
- Cretu AM, Mocanu L, Sora A, Nicolau AA (2021) A new technique of performing the cell block using egg whites. Ovidius University Annals of Chemistry 32(1): 46-52.
gynaecological: um escudo de 48.355 Casos. J Bras Patola Med Lab 38(2): 141-147.
minimum alcohol use: a cost-cutting measure for resource-limited settings. Cytopathology 21(4): 229-
development of the Papanicolaou stain method. Acta Cytol 61(4-5): 266-280.
Papanicolaou stain for the diagnosis of cervical cancer. Acta Cytol 60(1): 79-84.
-
Ana Maria Cretu*, Nicolau Anca Antonela, Liliana Seicaru, Miruna Cristian and Tase Geta Manuela. Development of a New Tehnique in Cytology, Protocol for Direct Staining in the Test Tube of Cervico-Vaginal and Samples. Arch Biomed Eng & Biotechnol. 8(1): 2025. ABEB.MS.ID.000679
-
Medicinal herb, Herbal ingredients, processed, Pattern identification, Chinese medicinal herbs, Traditional Chinese medicine, Olympic athletes, Including randomized trials, Medicinal herbs
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






