 Review Article
Review Article
A Pioneering Genome Editing Tool with Crispr-CAS9 Approach for Application in Cancer Therapy: Future Perspectives and Challenges
Kainat Ramzan1*, Imran Haider1, Maliha Sarfraz2, Ayesha Aslam1, Noor Zada khan3, Sonia1, Sabahat Tanveer1, Saira Ramzan4 and Hayat Ullah5
1Department of Biochemistry, University of Okara, Okara-56130, Pakistan
2Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad, Sub-campus Toba Tek Singh 36080, Pakistan
3Department of Microbiology, University of Peshawar, Peshawar, Pakistan
4Department of Zoology, Faculty of Life Science, University of Okara, Okara-56130, Pakistan
5Institute of Chemistry, University of Okara, Okara-56300, Panjab, Pakistan
Kainat Ramzan, Department of Biochemistry, University of Okara, Okara-56130, Pakistan
Received Date: July 08, 2024; Published Date: September 23, 2024
Abstract
Cancer is a rapidly progressive condition characterized by a loss of control over cell division and mortality, DNA alterations, impaired healing process, and other causes. The cancerous cell environment is crucial in disease progression since it contains a variety of immunological cells, both innate and adaptive, that contribute to the onset of the disease. Aside from the medicines that have been discovered, the inadequate medical effects of tumors can be overcome in the future by expanding genetic toolboxes. Immunotherapy has resulted in the modern era of cancer therapy however; it is still in its early stages and must be monitored to avoid problems. Subsequent genomic research and engineering may hasten the development of sophisticated technology. Prior research showed that genetic and mutant genes are directly associated with the incident, development, and medical prospects of cancerous cells. The progress of innovative gene modification tools, particularly the globally recognized clustered regularly interspaced short palindromic repeats (CRISPR-Cas9) platform, showed considerable prospective in overcoming medical constraints. The present study demands to offer an extensive review of potential cancer targets for CRISPR-Cas9 and underscore the clinical evidence supporting its potential application in cancer treatment. Moreover, the findings offer an enlarged perspective of the CRISPR-Cas9 clinical relevance as well as its limits. As a result, the study intends to highlight a prospective and futuristic immunotherapeutic technique in conjunction with established treatment procedures.
Keywords:Cancer immunotherapy; WHO; FDA; EMA; CART; Agrobacterium
Introduction
As of February 2017, the World Health Organization (WHO) released a fact sheet indicating that cancer was a significant cause of global mortality, responsible for 8.8 million deaths in the year 2015. This alarming statistic reveals that approximately one in every six deaths worldwide was directly linked to cancer [1-3]. In 2019, it was anticipated that 140,690 incidences of cancer will be confirmed, with a significant portion of these patients expected to face the ongoing challenges of the disease throughout their lives [4,5]. Cancer is a significant proliferative disease that involves an impairment of development and apoptotic control, DNA disruption, and an improper repairing process. Because it comprises lymphocytes (B and T-cells), a tumor context serves a significant part in disease progression. The substances formed by tumor proinflammatory cells are the most critical molecules for developing an association between inflammatory reactions, innate/adaptive immune response, and cancer [6-9].
In 1909, Paul Ehrlich proposed the concept of tumor immunology and developed antibodies that had possible ability to combat cancerous cells [10,11]. In 1950s, Burnet and Thomas proposed autoimmune surveillance concept, arguing that the immune response destroys a risk cells at the primary site before they grow into apparent cancer cells [12]. In 2001, Robert D Schreiber and his colleagues coined the word immunoediting to describe the process by which tumors are determined [13]. Immunotherapy has shown positive response in a variety of clinical studies whereby diverse exogenous manipulated interferons, cytokines, interleukins, and antibodies are used to induce a higher immune reaction than traditional approaches [14]. Globally, various cancer treating therapies, including as radiotherapy, surgery, and chemotherapy are currently recognized and widely used [15-18]. Although, directed therapy, photothermal (PT) and photodynamic therapy (PDT) are all being researched [19].
Given the use of such traditional treatments as bridges, there is a gap in treatment possibilities for patients relapse-free lifespan [20]. So, there is a need for advances in cancer treatment to counteract these limitations. Immunotherapy has grown as a new approaches in the modern scenario, and different kinds of drugs are being developed as illustrated in Figure 1 [21]. Immunotherapy is used in conjunction with conventional drugs or with adjuvants to form neoadjuvant treatments [21], and may assists in avoiding the risk of tumor relapse among individuals with preliminary tumors [22]. Combination chemotherapy used when a number of medicines is given during adjuvant treatment, while hormonal therapy is known to be effective in instances of cellular cancers i.e. breast cancer [23]. Tumor genesis necessitates a number of genes and epigenetic modifications [24,25].
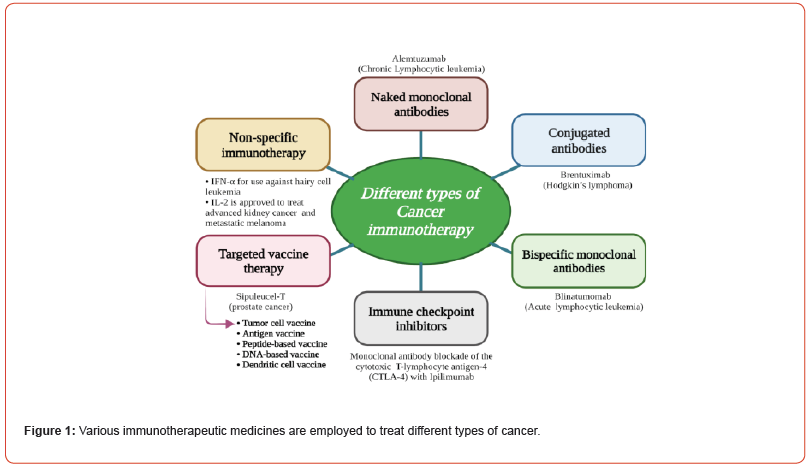
The WHO has forecasted a persistent increase in cancer mortality rates for the foreseeable future. By the year 2030, it is projected that around 13.1 million deaths worldwide will be attributed to cancer [1,26-28]. In 2021, the American Cancer Society (ACS) confirmed about 1.9 million individuals are expected to be screened for melanoma. Tragically, it is anticipated that around 608,570 deaths will be attributed to melanoma during that period [29-31]. Traditional tumor therapy can result in radiation attacks, toxic substances, and other negative responses, which may result in death; consequently we must expand current therapies to address possible cancer response [32,33]. Tumor immunotherapy aims to prevent tumor cell proliferation and infiltration by activating the immune cells [34]. The immune-mediated checkpoint inhibition (ICI), adaptive cellular therapy (ACT), cancer vaccines, oncolytic virus, dendritic cell (DC), and antibody-drug conjugate (ADC) therapies were all used for treating cancers. Furthermore, the usage of anti-CD19 chimeric antigen receptor (CAR) action proceeds a substantial progress on the cure of lymphatic lesions [35].
The FDA licensed anti-CD19 chimeric antigen receptor (CAR) T-cell therapies to serve treatment of both chronic B-cell-mediated malignancies and carcinomas in 2017, indicating a significant step forward in its curative potential [36,37]. Regina studied the progress of CAR-T therapy, including numerous approaches combining artificial genomes and multimodal ligands to combat antigenic evasion and influence the microenvironment of cancer cells [38,39]. Even so, immune treatment is generally limited for the preclinical phase due to immunosuppressant antagonists and cytokines release syndrome of CAR-T cells [40].
In 2018, James P. Allison was awarded the Nobel Prize in Physiology or Medicine for his pioneering discovery of cytotoxic T-lymphocyte-associated protein (CTLA-4), while Tasuku Honjo received the same prestigious award for his significant contribution in identifying programmed cell death protein 1 (PD- 1) or programmed cell death protein ligand 1 (PD-L1) [41- 44]. Immunotherapy offers a variety of drawbacks, the risk of disrupting immunological equilibrium by inducing an allergic reaction that is ineffective against healthy tissue. Common adverse effects of drugs and other disorders include persistent irritation, diarrhoea, and itchy skin [45]. In cancer surveillance, tumor cell-derived chemicals cause reactions by combining receptors on antigen-presenting cells (APCs) or by a unique effector role aimed at removing developing cancerous cells [46]. Tumor-associated antigens (TAAs) assists host immunity in detecting and eliminating malignancies that invade normal tissue [47,48].
Despite conventional therapy, immunotherapy remodels the microenvironment of cancer with chemokines, cytokines, and lymphocytes, which may result in significant effects and prevent relapse [49,50]. For in vitro or in vivo studies, a clinical trial has proved the beneficial effects of tumor therapy. A proper therapy regimen is determined by the origin and stage of the cancer [51,52]. The FDA has approved a wide range of prescribed drugs for use in oncology therapy. Over the past 30 years, Interleukin 2 for kidney cancer, pioneering monoclonal antibodies (mAb) for B-cell tumors, and the prostate cancer vaccination based on DC, CAR-engineered methods for B-cell lymphoma, and PD-L1 inhibitors for cancer have all been recognized to be used to treat cancer [53]. In latestage clinical trials, researchers continued to evaluate over forty antibodies in melanoma [54]. In 1988, Greg humanity approach contributed to the production of mAbs for the therapeutic use of various cancer [55]. The US FDA and the European Medicines Agency (EMA) obtained two vaccinations for late prostate tumors, Imylgic® (T-VEC) to accelerate cancer formation and Provenge® (Sipuleucel-T) to deliver the GM-CSF coding genes [56].
Currently, The FDA and EMA have authorized over 100 mAbs for therapeutic purpose of cancer and immunological and chronic inflammatory disorders [59,60]. Additionally, the FDA and EMA have both provided approval for a range of checkpoint antagonists, such as ipilimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, cemiplimab, as well as conventional chemotherapy agents like carboplatin, paclitaxel, and targeted therapy agents like bevacizumab and avelumab [61-63]. For a decade, immunoglobulins targeting immunoinhibitory molecules are frequently prescribed the therapeutic drugs [64]. Several antibodies and small compounds, notably; CD276, CD39, CD73, the A2A, and CD47 all are being tested in clinical studies to block immune regulatory receptors [65]. The US FDA has authorized Nivolumab, Pembrolizumab, and Cemiplimab antibodies as PD-1 agonists [66]. Many cancer treatment combos are now approved by the FDA, notably ipilimumab, pembrolizumab combined T-VEC, ipilimumab coupled with peptide vaccination, nivolumab with a peptide vaccine, pembrolizumab with virus vaccine, and ipilimumab with Sipuleucel-T in prostate cancer cases [58].
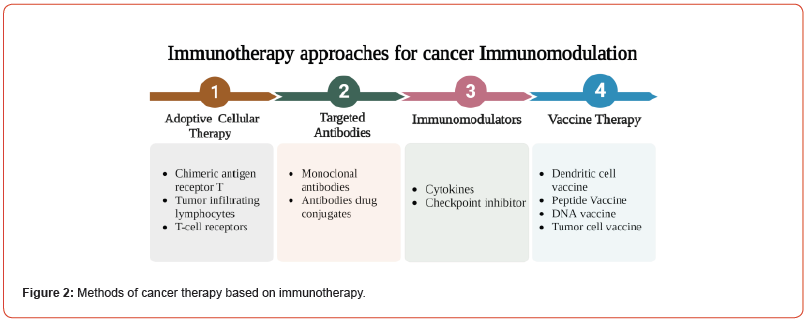
Tumor immunotherapy techniques are divided into four categories, as indicated in Figure 2: immune modulation, adoptive cellular treatment, targeted antibodies, and tumor vaccinations [57]. Furthermore, numerous drugs have an effect on immune regulation; the primary process relies on the activation of APCs to T lymphocytes, with the result that cancer cells are destroyed. The cytokines are the most often used as immune stimulation drugs, with many cytokine-based modulators approved for cancer therapy. For instance, Proleukin® (Aldesleukin) is a synthetic interleukin 2 generated by gene recombination for bladder cancer and melanoma treatment [67]. A CTLA-4 agonist Yervoy® (Ipilimumab) designed to suppress the expression of CTLA-4 on T lymphocytes, also employed to treat metastatic melanoma, rheumatoid arthritis (RA), and colitis with ulcers [68]. The Atezolizumab (Tecentriq®), a PD-L1 inhibitory agents used to treat lung and urothelial carcinoma [69].
Further studies for novel checkpoint inhibitors as are currently being planned. In 1-phase cases, antibodies targets to CD47, CD73, A2Ar, and TIM-3 proved to be effective in the solid tumor therapy [70]. Tumor-Infiltrating Lymphocyte (TIL) represents a form of immunotherapy that uses lymphocytes (T cells) found in the malignant tissue targeted, amplified, and re-infused back into the patient’s body with a sufficient proportion. Given its obvious potential, the TIL method has a few drawbacks. For example, while T cells proliferate in vitro, this may not be the case in patients. A genetically engineered T cell receptor technique based on peripheral cell lines was developed to solve such issue [71,72]. Both TIL and TCR methods may be employed to target malignant cells exhibiting antigens. The T lymphocytes recognize malignancy that is major histocompatibility complex (MHC) independent in the CAR-T pathway. Example of personalized therapy in action, and the FDA and EMA confirmed the drugs Kymriah® and Yescarta® for myeloma treatment [73].
The mAbs are immunoglobulins (Ig) that usually have two Fab terminals that attaches to targets and an Fc terminus that binds to receptors on the exterior of lymphocyte [74-77]. The finding of antigens specific to tumors raised concerns regarding antibodies. As a result, Rituximab was the first mAb designed to target CD20 activity on B cells in non-Hodgkin lymphoma (NHL). Following this, anti-HER2 Trastuzumab and anti- VEGF Bevacizumab were approved in the therapy of breast cancer, as also anti-EGFR Cetuximab and anti-HER2 Trastuzumab for the cure of colorectal cancer [78,79]. In 2013, an antibody-drug conjugate called Kadcyla is originally authorized as the therapeutic drug of HER2-sensitive advanced cancers [80]. Active therapy has been shown by the use of vaccines in tumor immunotherapy [81]. The first commercial DC based vaccination licensed by the FDA was sipuleucel-T (Provenge) designed for prostatic cancer treatment [82], and Talimogene laherparepvec (T-VEC) vaccine serves for treating cancers [83,84]. Traditional therapy entails either providing a gene whose activity can disrupt the synthesis of tumor-promoting genes or inserting a functional gene to compensate for an inactive gene. As a result, greater genetic locus specificity is necessary to generate superior and more secure outcomes [85,86].
For over 20 years, tumor-suppressing genes, metabolismrelated DNA, and markers for chemotherapy and radiationresistant genes have been identified and altered by a combination of the CRISPR-Cas9 tool to suppress the onset and malignancy progression [87-90]. In addition, immunotherapy has marked the dawn of an era of progress in cancer care, it is effective only in certain types of tumors and is tolerated by a small percentage of cancer patients [91]. Technologies for editing genomes, such as zinc-finger endonucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the CRISPR-Cas9 approach are utilized to target, knockout, insert, and modify specific genes, aiming to eradicate cancer [92]. The ZFNs are hybridized that contain two dimer subunits (DNA binding and cleavage domain), a highly specialized pair of molecule scissors, and FokI nuclease from Flavobacterium Okanokotes fused into double stranded breaks (DSBs) that could be corrected through mechanism of DNA repairing.
The TALENs repeats produced by Xanthomonas bacteria are made up of a FokI endonuclease that fuses with a variable DNAbinding target to cause DSBs [93-96]. Because of its simplicity, feasibility, and potential diversity, CRISPR-Cas9 approach is preferred for modifying genes. As a result, CRISPR-Cas9 gene editing could offer a novel strategy for cancer immunotherapy [97- 99]. Aside from the expanding area of the base and prime editing, the ability to create DSBs at unique and precise loci that affects the efficacy and feasibility of DNA modification [100]. As a result, impaired ends are fixed by either using non-homologous end joining (NHEJ) and homologous recombination repair (HDR) mechanism [97,101]. The current study emphasizes the implementation of CRISPR-associated approaches as a viable means of combating cancer. Moreover, we evaluate present challenges as well as future prospects. However, the potential for negative effects restricts its application in medicine, necessitating an effective ethical evaluation. The biological underpinnings of the CRISPR-cas9 technique are examined in this publication, along with the benefits and drawbacks of cancer research endeavors.
Principal action of the CRISPR-Cas9 method
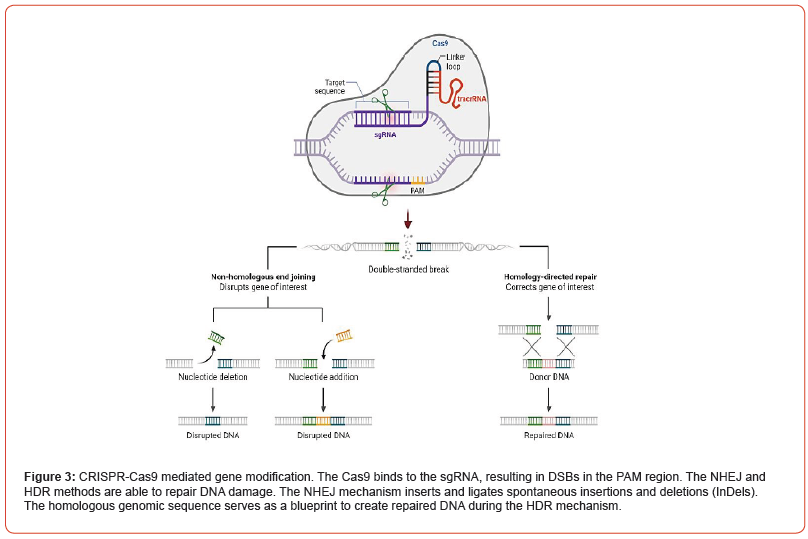
The discovery of DNA modification approach in E. coli was originally documented in 1987. Subsequently, these distinct repetitive sequences were also observed in the genomes of various microbial and archaeal organisms [102-104]. In 2020, Emmanuelle Charpentier and Jennifer Doudna were awarded the Nobel Prize to honor their significant advances to the CRISPR-Cas9 technology. This prestigious recognition came shortly after the identification of the essential chemical modules of the CRISPR-Cas9 method. Their pioneering research upon DNA modification typically stated to as the genetic scissors, earned them a joint Nobel Award in Physiology and Medicine [104-107]. Until their function was unknown, when the spacer segments were found to comparable sequences found in bacteria, archaeal viruses, and plasmids. If these repeats are matches, the invading exogenous DNA cannot infect bacterial cells, showing that they serve as a bacterial defensive strategy [108]. As shown in Figure 3, the CRISPR-Cas9- mediated immune response occurs in three stages: acquisition, transcription, and interference [109-111]. Bacteria have developed a method for collecting and integrating viral DNA snippets from invaders into their own genes, ending in the formation of CRISPR arrays. This unique mutation allows bacteria to anticipate viral interactions. In reply to an attack, bacteria use CRISPR array-derived fragments of RNA to execute a targeted attack on the viral genome, providing a defense mechanism against viral infections [112].
The microorganisms use Cas9 or a related enzyme to trim the genetic region, decreasing the virus survival and adverse activity. The bacterial CRISPR-Cas9 system consists of up of a pair of distinct segments of RNA called the mature RNA (crRNA) and the trans-activating crRNA (tracrRNA) [86,115,116]. This tracrRNA is combined with the crRNA, an active guide RNA (gRNA) is generated. The tracrRNA span contains 3 stem-loop chains and 1 anti-repetitive domain, while the crRNA just has guide and repeat segments. By combining amino acids with the nucleotide targeting region, the Watson and Crick guiding region creates the gRNA and DNA heteroduplex. Notably, the complex structure is directed to identify viral strands by the crRNA spacer segment rather than the tracrRNA component of the guide, with which Cas9 interfaces [115, 117, 118]. Indeed, crRNA and tracrRNAs collaborate for producing the Cas9- RNA protein complex, leading to DSBs in the viral genome. The main advantage is how the gRNA may be adjusted irrespective of the endonuclease, allowing CRISPR to be enhanced as a DNA editing approach with infinite targeted potential and significant precision [119-121]. Despite typically repeated sequences in DNA, nonrepeating sections of genetic material known as spacers from infectious viruses define CRISPR repeat clusters [122,123].
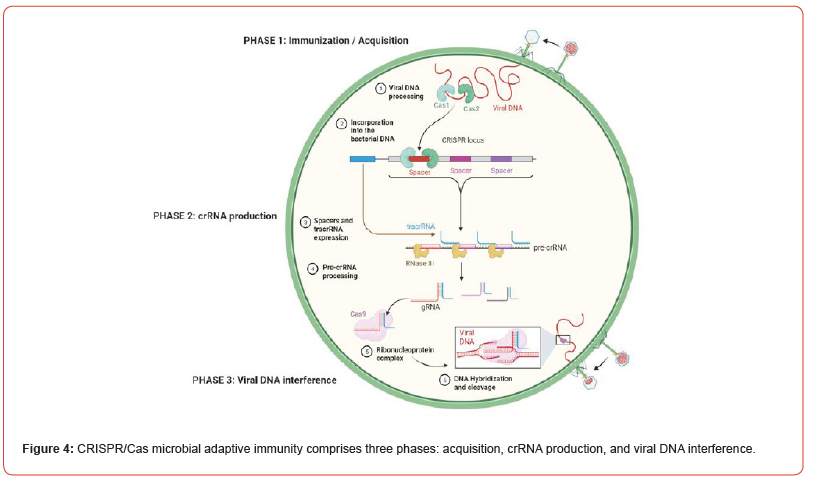
However, spacer repeats and protospacer-adjacent motif (PAM) series chosen by the gRNA are short chains of DNA (typically 2-6 bp) positioned 5′-NGG-3′ below from the targeted sites [124,125], resulting in blunt sequence ended. As seen in Figure 4, DSBs activate cellular repair pathways, most notably NHEJ or HDR repair [126,127]. The PAM binding cleaves the invading phage DNA, creating a DSB and interfering with phage expression and replication. The CRISPR method couples the Cas9 enzyme, with a sgRNA that functions similarly to the crRNA and tracrRNA tandem in microbes. While sgRNA is critical in determining nuclease selectivity and snipping activity [128]. Two nuclease domains are involved in the cleavage: the HNH domain (cleaves the desired strands), and the RuvC domain (divides the contrasting side). The Type-II attract the attention of researchers due to their small level of complexity [129,130]. In 2013, after Cas9 optimized to target human coding and nuclear localization signals were included, CRISPR-Cas9 was initially employed among mammals and mouse embryonic cells [131,132]. Thus, CRISPR-Cas9 seemed as a novel dynamical technique for modifying genes since by changing only the crRNA pattern and tracrRNA and endonuclease, unaffected. This method avoids the challenging issue of protein production, resulting in affordable and faster. In mammals, DSBs are repaired by NHEJ after Cas9 PAM identification and fragmentation. This might lead to an apparent reading frameshift mutation and a decrease in the yield of the altered chromosomal region [86,133,134].
Animal and Human biology of CRISPR-Cas9 methods
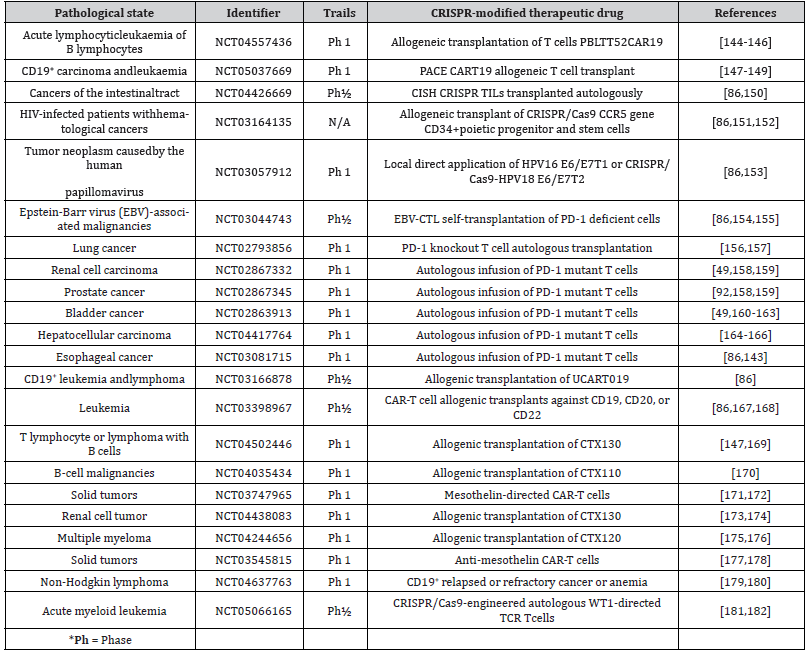
The CRISPR-Cas9 has enormous potential for treating and diagnosing cancer, involving 1) the adoption of CRISPR-Cas9- based screening tools SHERLOCK and DETECTR for tumor testing, 2) offering TCR knockout (KO) CAR-T cells, 3) KO of antagonistic receptors such as PD-1 and LAG-3 to boost tumor immunotherapy competence, 4) eradication of cancer-causing virus-like HPV, and 5) through eliciting genetic polymorphisms produce in vivo cancer models [139,140]. Moreover, it could be employed for producing and replicating alterations, by screening the growth of tumor formation in model animals [92]. The pups and hogs are included in the group of genome altering breeds for hereditary and medical studies owing to a scarcity of knowledge about embryological stem cells and the HR viability [141]. Over 25 studies are being carried out to look into the dependability and effectiveness of employing CRISPR-Cas9 for treating cancer, with significant advances in the CRISPR concept targeted at overcoming any difficulties that may hamper its clinical application [86]. As shown in Table 1, multiple studies are underway to examine the potency and anticancer effects of CRISPR-Cas9 in various cancers [86,142,143].
Table 1:Overview of cancer research studies involving CRISPR approach.
Furthermore, there is significant promise in establishing inherited disorders across the model animals, and model simulation of polymorphism in CRISPR-Cas9 lineage, which could lead to advancements in DNA therapy and renewing healthcare [183]. The invention of tumor-specific toolkits boosts the potency of DNA therapy through the regulation of tumor inhibitor and oncogenic activity [184]. Human DNA editing using CRISPR-associated Cas9 mediators, as well as gene screens and immune-based therapeutic applications in disease biology [185,186]. The mutagenic method also enables for forecasting the markers sensitive to insignificant compounds, such as Schlafen Family Member 11 (SLFN11) that responds to poly-ADP ribose polymerase and serves against lung cancer diagnosis [187]. The study of polymorphism in EGFRtyrosine antagonists resulted in the discovery of actual variants and the defeat of specific susceptibility [188]. Genetically altered mice got infected with adeno-associated virus vectors containing gRNAs targeted at the p53 and RB1 tumor inhibitor variants in small cell lung cancer (SCLC). The modeled species revealed clinical traits similar to human cancer, mouse genes suppressed by specific changes in P53 and RB1 was used as potential human target therapy [92].
Further, gRNAs were specifically created to target other genes, so as better comprehend their association with the disorder and pathology. Biological luminescence microscopy showed significant luciferase expression in gRNA-107 and 130 cells infected 6 months after tumor formation, indicating their significance in early cancer spread. As such, CRISPR was effective at replicating cancer in animal models and finding variants in novel traits that cause small lung cancer metastases [189]. In addition, GeCKO allowed the discovery of changes and the recognized the collaborative role of potential Tp53 and the KRAS cancer genes in KrasG12D rendered rat early fibroblasts, resulting in improved knowledge of early carcinomas in mice [190]. A human GeCKO library screened 6 potential cisplatin resistance genes for cervical cancerous cells, with identified genes (SULF1, ZNF587B), whose absence elevated cisplatin vulnerability [191]. Another study, scientists examined the FRK tumor gene significant in H1299 lung cancerous cell line, revealing that FRK deletion inhibited epidermal to mesenchyme shift cell growth and cancer formation [192]. The TP53KO cells found for diagnosis drugs and P53 tumor gene [141]. Because of its capacity to alter not only short segments of genes but also large sections in model organisms, the CRISPR-Cas9 strategy can be utilized to produce abnormal chromosomes [193]. Most of the aforementioned cases indicated that CRISPR- Cas9 potency in cancer modeling and in vitro research, as well as its potential applications for gene therapies. Though this strategy is still not widely used, the literature review is likely to increase attention to its worth for health care and may offer greater insight into the molecular etiology of a range of disorders. Thus, Table 1 displays instances of active clinical research including therapeutic drugs with a CRISPR-Cas9 component [86, 194].
Implications of CRISPR-Cas9 in infectious and inherited diseases
During the pathogenic activity, CRISPR-Cas9 has become potential to enhance the release of either DNA or RNA virus from infected cells. CRISPR-Cas9 offers effective genomic treatment for fighting mammalian infections by targeting several stages of the virus lifecycle [140]. To modify infectious viruses, efficient antiviral approaches based on CRISPR-Cas9 were used, and known to be highly effective in fighting viruses that cause immune deficiency such as HIV, and hepatitis. The findings indicate a promising approach to viral manipulation and offer prospective responses for dealing with viral diseases [195,196]. DNA viruses such as Kaposi’s sarcoma herpesvirus (KSHV), Epstein-Barr virus (EBV), human papillomavirus (HPV), hepatitis B virus (HBV), and Simian virus 40 (SV40) are frequently attributed for cancer development. Aside from these DNA viruses, certain RNA viruses, such as human T-lymphotropic virus-1 (HTLV-1) and hepatitis C virus (HCV), have been linked to cancer [197,198]. The CRISPR/Cas9 combined with additional therapies, including the NU7026 P mediator, can effectively eradicate the disease-causing HBV gene [199]. In this context, in vitro ablation of the HPV16-E7 CRISPR-Cas pathway raised cell death and delayed the formation of cervical carcinoma strains while having no consequence against HPV cells. The absence of E7 DNA improved the production of the tumor suppressor protein retinoblastoma, implying that E7 might be targeted for DNA editing methods for treating cervical carcinoma [200].
Furthermore, it was shown that integrating CRISPR-Cas9 with immune-regulating drugs, which are FDA authorized to enhance the cancer treating purposes [201,202]. Combining CRISPR-Cas9 enabled ablation of the HPV16 gene with a PD1 inhibitor enhanced survival rates and tumor progression. Furthermore, medication raised the level of APCs, CD8+ cytotoxic, and CD4+ T helper cells in the cancerous tissue, resulting in strong anticancer effects in mice [140]. CRISPR/Cas9 technology has demonstrated its capability in correcting defective genes associated with various diseases. Examples include the correction of genes like DMD in Duchene muscular dystrophy, CFTR in cystic fibrosis, factor IX in hemophilia B, and several others linked to conditions such as Dementia, Huntington’s, and Parkinson’s diseases, Tay-Sachs disease, and fragile X syndrome. This breakthrough technology holds immense potential for gene correction and treatment of these disorders [86,91,121,140,203]. Clinical trials are currently evaluating the delivery systems for CRISPR-Cas9 monitored by the transplantation of these cells to enhance their anti-tumor activity in patients. Chira and colleagues have provided comprehensive insights into CRISPR/ Cas9 delivery methods in a book section, shedding light on this area of research [86].
According to Mehta, et al. [263], presume that CRISPR-Cas9 has significant inhibitory effects due to virus escape and rapid growth. To conquer such obstacles, the development of an effective, reliable, and widely accepted CRISPR/Cas13a technique is crucial. Recently, CRISPR/Cas13a exhibits the greatest scalable RNA targeted technology for RNA modifications and RNA virus targeting among the three Cas13 protein families [264]. The Cas13a protein are used for nucleic acid identification, RNA knockdown, and transcript tracking in human and plant cells [265]. By using basic [266] and multiplexed gene editing [267], CRISPR has raised plant disease resistance and also many other attributes such as yield [268], quality of crop [269] tolerance to biotic and abiotic stressors, and sperm sterility. Particularly, many multiplexed genome editing tools may alter many genes at the identical time are now available [270].
The CRISPR may offer remedies for COVID-19 patients alongside to having diagnostic significance. For SARS-CoV-2 DETECTR, a CRISPR Cas12-based test for the detection of coronavirus was recently developed.
The VI-D CRISPR-Cas13d variant derived from Ruminococcus flavefaciens was chosen for its tiny size, allowing for facile packaging in viral carriers, useful sensitivity, and robust catalytic action in human cells [113,271]. Despite the fact that SARS-CoV-2 has an extremely high mutation and recombination rate, this technique evolved to concurrently target multiple loci for RNA depletion, paving the door for an essential pan-coronavirus targeting approach. With this advancement, the CRISPR-Cas system might be re-implemented to serve its initial intent as a virus-fighting action to aid in the the outbreak response [272, 273].
Future Perspective
Over decades, CRISPR-Cas9 genetic modification was successfully entered the preclinical and clinical phases as a disease therapeutic strategy. As gene-editing methods evolved and new drug for diseases have been uncovered, clinical translation and practical research in the genomic area has grown. CRISPR-Cas9 is known to be highly effective not only in insects and plant life, but also in animals and human beings, and it has a promising future in cancer biology since it is a versatile, easy, convenient, and efficient technique. The method brings a novel approach to cancer treatment by enabling previously unattainable genomic modifications in target cells. A particular gene variation raised cancer migration, invasion, and angiogenesis, which may be dealt with through genetic modifications. The CRISPR-Cas system is now being used in vivo to combat cancer and immunological problems. Research studies to validate the effects of CRISPR- Cas9 are underway, with promising results. There were investigations with just a few of patients and minimal afterward, and further in-vivo studies are anticipated.
Meanwhile, continuous surveillance is required to validate the effects and identify any unanticipated problems. Expansion and refinement of Cas9-based modification may accelerate research toward medicinal uses and provide a number of therapy options for a variety of diseases, including cancer. Regardless of how CRISPR/Cas9 is employed, it shows promise for gene mutationrelated cancer therapy; nevertheless, challenges such as off-target and ethics must be addressed. Scientists are bound to follow the global consensus and strive to positively develop society through technology.
Researchers have effectively created Cas9 variations that are specifically targeted to minimize off-target effects (OTEs) while keeping high editing efficacy. According to the theory, the high affinity between Cas9 nuclease and the specific DNA could contribute to OTEs. By adding mutations to four critical residues responsible for direct hydrogen linkage between Cas9 and the phosphate core of the target DNA, the SpCas9-HF1 showed no detectable off-target activity when compared to wild-type SpCas9. These Cas9 variant production advances have the prospective to improve the accuracy and security of CRISPR-based genetic modification. The Cas9 enzyme has the ability to help manage the off-target features and undesired side effects of the CRISPR system. Researchers should look into a number of physical or chemical substances, such as tetracycline and doxycycline, as a potential method for developing Cas9 expression. Furthermore, a variety of online tools are accessible to assist researchers in creating sgRNA with low off-target effects in order to increase selectivity for human gene modification.
Currently, a few CRISPR-mediated gene modifications, such as shorter sgRNA, Cas9 nickase, and Fok1- dCas9 interaction, have shown promise in reducing off-target effects. The Cas9 massive size poses packing challenges, particularly in low immunogenic AAV vectors, which are commonly used in gene delivery. According to a new study, the extended attachment of Cas9 nuclease to DSBs restricts repair protein access to the target location, lowering repair efficiency. Nonetheless, the translocating RNA polymerase might be used to separate the template-bound Cas9 only if the DSBs are found in a precise direction by the RNA polymerase.
Furthermore, using the current CRISPR/Cas9 technology, some carcinogenic genomic variations, such as chromothripsis, aneuploidy, and LINE-1-mediated genome modification, remain difficult to cure. However, the mutagenesis efficiency of CRISPR/ Cas9 should be boosted in the near future by creating more potent Cas9 and devising effective delivery systems with dominant sgRNA. In model organisms and humans, the CRISPR/Cas9 technology allows for precise editing of a target sequence for therapeutic research. It is also potentially possible to use it to treat viral, inherited, and malignant conditions. Recent research has shown that Cas9 ribonucleoproteins (RNPs) are increasingly used as a viable alternative to plasmid vectors for delivering the CRISPR reagent into target cells. This method offers notable advantages, including improved efficiency, a shorter duration of Cas9 activity, and the elimination of vector sequence integration. However, it is important to note that this delivery approach does not have a direct effect on addressing chromosomal rearrangements. While Cas9 RNPs provide benefits in terms of delivery and reducing integration concerns, addressing chromosomal rearrangements may require additional strategies in the context of CRISPR-based applications.
Concluding Remarks
It is concluded that new techniques for overcoming the gaps surrounding the grey areas in Immunotherapy can be developed with the intervention of cost-effective and promising therapies. Furthermore, as tactics and technologies evolve, immune cells can lead to the creation of cost-effective immunotherapeutic treatments, which can then be used to generate personalized medicine based on patients’ tumor immune profiles. Despite the fact that adjuvant therapy and other immunizations are effective for the therapy of metastatic tumors, there is still a big opportunity to develop vaccines with minimal side effects. Aside from the traditional treatments of surgery, radiation, and chemotherapy, cancer immunotherapies are predicted to emerge as one of the most effective therapeutic choices available. This has also fueled conventional ways to boost the chance of long-term tumor decrease in cancer patients, leading to significant therapy alternatives.
References
- Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA: a cancer journal for clinicians 73(1): 17-48.
- Saeed S, Khan JA, Iqbal N, Irfan S, Shafique A, et al. (2019) Cancer and how the patients see it; prevalence and perception of risk factors: a cross-sectional survey from a tertiary care centre of Karachi, Pakistan. BMC public health 19(1):360.
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1): 7-34.
Hosseini SA, Jouneghani AS, Ghatrehsamani M, Yaghoobi H, Elahian F, et al. (2022) CRISPR/Cas9 as precision and high-throughput genetic engineering tools in gastrointestinal cancer research and therapy. Int J Biol Macromol. - Zhao G, Zhu S, Zhang F, Zhang X, Zhang X, et al. (2023) Global Burden of osteoarthritis associated with high body mass index in 204 countries and territories, 1990–2019: findings from the Global Burden of Disease Study 2019. Endocrine 2023 79(1): 60-71.
- Alhmoud JF, Woolley JF, Al Moustafa A-E, Malki MI (2020) DNA damage/repair management in cancers. Cancers 12(4): 1050.
- Chaudhry G-E-S, Md Akim A, Sung YY, Sifzizul TMT (2022) Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Frontiers in Pharmacology 13: 842376.
- Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77: 1745-1770.
- Dehghani T, Shahrjerdi A, Kahrizi MS, Ravandeh S, Merza MS, et al. (2023) Targeting programmed cell death protein 1 (PD-1) for treatment of non-small-cell lung carcinoma (NSCLC); the recent advances. Pathol Res Prac 246: 154470.
- Ribatti D (2017) The concept of immune surveillance against tumors: The first theories. Oncotarget 8(4): 7175-7180.
- Galon J, Bruni D (2020) Tumor immunology and tumor evolution: intertwined histories. Immunity 52(1): 55-81.
- Ostrand-Rosenberg S (2008) Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 18(1): 11-18.
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, et al. (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410(6832):1107-1111.
- Gholizadeh Z, Tavakkol‐Afshari J, Nikpoor AR, Jalali SA, Jaafari MR (2018) Enhanced immune response induced by P5 HER2/neu‐derived peptide‐pulsed dendritic cells as a preventive cancer vaccine. J Cell Mol Med 22(1): 558-567.
- Wyld L, Audisio RA, Poston GJ (2015) The evolution of cancer surgery and future perspectives. Nature reviews Clinical oncology 12(2): 115-124.
- Schaue D, McBride WH (2015) Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol 12(9): 527-540.
- Galmarini D, Galmarini CM, Galmarini FC (2012) Cancer chemotherapy: a critical analysis of its 60 years of history. Crit Rev Oncol Hematol 84(2): 181-199.
- A Baudino T (2015) Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol 12(1): 3-20.
- Li X, Lovell JF, Yoon J, Chen X (2020) Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol 17(11): 657-674.
- Payne KK, Toor AA, Wang X-Y, Manjili MH (2012) Immunotherapy of cancer: reprogramming tumor-immune crosstalk. Clin Dev Immunol 2012: 760965.
- Bondhopadhyay B, Sisodiya S, Chikara A, Khan A, Tanwar P, et al. (2020) Cancer immunotherapy: A promising dawn in cancer research. American Journal of Blood Research 10(6): 375.
- Chew HK (2001) Adjuvant therapy for breast cancer: who should get what?. Western Journal of medicine 174(4): 284.
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, et al. (2007) Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450(7171): 903-907.
- Podlaha O, Riester M, De S, Michor F (2012) Evolution of the cancer genome. Trends in Genetics 28(4): 155-163.
- Huang W, Yan Y, Liu Y, Lin M, Ma J, et al. (2020) Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin α2β1. Signal Transduct Target Ther 5(1): 39.
- Boyle P, Levin B (2008) World cancer report 2008: IARC Press, International Agency for Research on Cancer.
- Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3(1): 7.
- Mattiuzzi C, Lippi G (2019) Current cancer epidemiology. J Epidemiol Glob Health 9(4): 217-222.
- Siegal R, Miller KD, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1): 9-29.
- Statistics NCfH (2023) Health, United States, 2020-2021.21
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, et al. (2021) Cancer statistics for the year 2020: An overview. Int J Cancer 149(4): 778-789.
- Deng X, Shao Z, Zhao Y (2021) Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Advanced Science 8(3): 2002504.
- Nyström H (2021) Extracellular matrix proteins in metastases to the liver–Composition, function and potential applications. Semin Cancer Biol 71: 134-142.
- Yang Y (2015) Cancer immunotherapy: harnessing the immune system to battle cancer. The Journal of clinical investigation 125(9): 3335-3337.
- Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, et al. (2016) CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest 126(6): 2123-2138.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, et al. (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26): 2531-2544.
- Frigault MJ, Maus MV (2020) State of the art in CAR T cell therapy for CD19+ B cell malignancies. J Clin Invest 130(4): 1586-1594.
- Rezaei R, Esmaeili Gouvarchin Ghaleh H, Farzanehpour M, Dorostkar R, Ranjbar R, et al. (2022) Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy. Cancer Gene Ther 29(6): 647-660.
- Young RM, Engel NW, Uslu U, Wellhausen N, June CH (2022) Next-generation CAR T-cell therapies. Cancer Discovery 12(7): 1625-1633.
- Sharaf Z, Behzadifar M, Behzadifar M, Fitzmaurice C, Abate D (2021) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. Global Burden of Cancer
- Altmann DM (2018) A Nobel Prize‐worthy pursuit: cancer immunology and harnessing immunity to tumour neoantigens. Immunology 155: 283-284.
- Furukawa F (2018) The Nobel Prize in Physiology or Medicine 2018 was awarded to cancer therapy by inhibition of negative immune regulation. Trends in Immunotherapy 2(1).
- Dahl O, Brydøy M (2018) The pioneers behind immune checkpoint blockers awarded the Nobel Prize in physiology or medicine 2018. Acta Oncol 58(1): 1-8.
- Smyth MJ, Teng MW (2018) 2018 Nobel Prize in physiology or medicine. Clinical & translational immunology 7(10).
- Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M (2017) Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pacific journal of oncology nursing 4(2): 127-135.
- Nayak DA, Binder RJ (2022) Agents of cancer immunosurveillance: HSPs and dsDNA. Trends Immunol 43(5): 404-413.
- Whiteside TL (2010) Immune responses to malignancies. Journal of Allergy and Clinical Immunology 125(2): S272-S283.
- Kim SK, Cho SW (2022) The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Frontiers in Pharmacology 13: 868695.
- Dutta S, Ganguly A, Chatterjee K, Spada S, Mukherjee S (2023) Targets of Immune Escape Mechanisms in Cancer: Basis for Development and Evolution of Cancer Immune Checkpoint Inhibitors. Biology 12(2): 218.
- Zhang W, Li S, Li C, Li T, Huang Y (2022) Remodeling tumor microenvironment with natural products to overcome drug resistance. Front Immunol 13: 1051998.
- Zhang Y, Wu L, Li Z, Zhang W, Luo F, Chu Y, Chen G (2018) Glycocalyx-mimicking nanoparticles improve anti-PD-L1 cancer immunotherapy through reversion of tumor-associated macrophages. Biomacromolecules 19(6): 2098-2108.
- Akkın S, Varan G, Bilensoy E (2021) A review on cancer immunotherapy and applications of nanotechnology to chemoimmunotherapy of different cancers. Molecules 26(11): 3382.
- Kuai R, Yuan W, Son S, Nam J, Xu Y, et al. (2018) Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv 4(4): eaao1736.
- Merino M, Contreras A, Casares N, Troconiz IF, Ten Hagen TL, et al. (2019) A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomedicine 17: 13-25.
- Buss NA, Henderson SJ, McFarlane M, Shenton JM, De Haan L (2012) Monoclonal antibody therapeutics: history and future. Curr opin pharmacol 12(5): 615-622.
- Slaney CY, Kershaw MH (2020) Challenges and opportunities for effective cancer immunotherapies. Cancers 12(11): 3164.
- Tran L, Xiao J-F, Agarwal N, Duex JE, Theodorescu D (2021) Advances in bladder cancer biology and therapy. Nat Rev Cancer 21(2): 104-121.
- Zhao J, Chen Y, Ding Z-Y, Liu J-Y (2019) Safety and efficacy of therapeutic cancer vaccines alone or in combination with immune checkpoint inhibitors in cancer treatment. Front pharmacol 10:1184.
- Kaplon H, Muralidharan M, Schneider Z, Reichert J (2020) Antibodies to watch in 2020. Mabs 12(1): 1703531.
- Kaplon H, Reichert JM (2021) Antibodies to watch in 2021. Mabs 13(1): 1860476.
- Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB (2020) Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 12(3): 738.
- Robert C (2020) A decade of immune-checkpoint inhibitors in cancer therapy. Nature communications 11(1): 3801.
- Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, et al. (2021) FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin Cancer Res 27(7): 1836-1841.
- Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Frontiers Oncology 8: 86.
- Chrétien S, Zerdes I, Bergh J, Matikas A, Foukakis T (2019) Beyond PD-1/PD-L1 inhibition: what the future holds for breast cancer immunotherapy. Cancers 11(5): 628.
- Huang Q, Zheng Y, Gao Z, Yuan L, Sun Y, Chen H (2021) Comparative efficacy and safety of PD-1/PD-L1 inhibitors for patients with solid tumors: a systematic review and bayesian network meta-analysis. J Cancer 12(4): 1133.
- Quijano-Rubio A, Ulge UY, Walkey CD, Silva D-A (2020) The advent of de novo proteins for cancer immunotherapy. Curr Opin Chem Biol 56: 119-128.
- Sun L, Chen L, Li H (2019) Checkpoint-modulating immunotherapies in tumor treatment: Targets, drugs, and mechanisms. Int Immunopharmacol 67: 160-175.
- Bernard-Tessier A, Bonnet C, Lavaud P, Gizzi M, Loriot Y, Massard C: Atezolizumab (Tecentriq®) (2018) Activity, indication and modality of use in advanced ormetastaticurinary bladder carcinoma. Bulletin du Cancer 105(2): 140-145.
- Marin-Acevedo JA, Kimbrough EO, Lou Y (2021) Next generation of immune checkpoint inhibitors and beyond. Journal of hematology & oncology 14(1): 45.
- Zhao Y, Deng J, Rao S, Guo S, Shen J, et al. (2022) Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: progressions and challenges. Cancers 14(17): 4160.
- Hulen TM, Chamberlain CA, Svane IM, Met Ö (2021) ACT up TIL now: the evolution of tumor-infiltrating lymphocytes in adoptive cell therapy for the treatment of solid tumors. Immuno 1(3): 194-211.
- Zheng P-P, Kros JM, Li J (2018) Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts. Drug discov Today 23(6): 1175-1182.
- Janeway Jr CA, Travers P, Walport M, Shlomchik MJ (2001) The structure of a typical antibody molecule. In: Immunobiology: The Immune System in Health and Disease 5th edition. Garland Science.
- Wang S (2011) Advances in the production of human monoclonal antibodies. Antibody Technology Journal 1: 1-4.
- Pantaleo G, Correia B, Fenwick C, Joo VS, Perez L (2022) Antibodies to combat viral infections: development strategies and progress. Nat Rev Drug Discov 21(9): 676-696.
- Quinteros DA, Bermúdez JM, Ravetti S, Cid A, Allemandi DA, et al. (2017) Therapeutic use of monoclonal antibodies: General aspects and challenges for drug delivery. In: Nanostructures for Drug Delivery. Elsevier: 807-833.
- Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9(3): 34.
- Lu R-M, Hwang Y-C, Liu I-J, Lee C-C, Tsai H-Z, et al. (2020) Development of therapeutic antibodies for the treatment of diseases. Journal of biomedical science 27(1): 1-30.
- Chen L, Wang L, Shion H, Yu C, Yu Y, et al. (2016) In-depth structural characterization of Kadcyla® (ado-trastuzumab emtansine) and its biosimilar candidate. MAbs 8: 1210-1223.
- Keshavarz-Fathi M, Rezaei N (2019) Chapter 2-Immunotherapeutic approaches in cancer. Vaccines for Cancer Immunotherapy Rezaei N, Keshavarz-Fathi M (Eds) 2019: 19-44.
- Handy CE, Antonarakis ES (2018) Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future oncol 14(10): 907-917.
- Raman SS, Hecht JR, Chan E (2019) Talimogene laherparepvec: review of its mechanism of action and clinical efficacy and safety. Immunotherapy 11(8): 705-723.
- Speeckaert R, van Geel N (2017) Vitiligo: an update on pathophysiology and treatment options. American journal of clinical dermatology 18(6): 733-744.
- Gonçalves GAR, Paiva RdMA: Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo) 2017, 15(3): 369-375.
- Chira S, Nutu A, Isacescu E, Bica C, Pop L, et al. (2022) Genome Editing Approaches with CRISPR/Cas9 for Cancer Treatment: Critical Appraisal of Preclinical and Clinical Utility, Challenges, and Future Research. Cells 11(18): 2781.
- Chen X, Liu Y, Zhang Q, Liu B, Cheng Y, et al. (2021) Exosomal long non-coding RNA HOTTIP increases resistance of colorectal cancer cells to mitomycin via impairing MiR-214-mediated degradation of KPNA3. Frontiers in Cell and Developmental Biology 8: 582723.
- Chen M, Mao A, Xu M, Weng Q, Mao J, et al. (2019) CRISPR-Cas9 for cancer therapy: Opportunities and challenges. Cancer lett 447: 48-55.
- Zhang H, Qin C, An C, Zheng X, Wen S, et al. (2021) Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Molecular Cancer 20: 1-22.
- Rodríguez TC, Dadafarin S, Pratt HE, Liu P, Amrani N, et al. (2021) Genome-wide detection and analysis of CRISPR-Cas off-targets. Prog Mol Biol Transl Sci 181: 31-43.
- Chen X-Z, Guo R, Zhao C, Xu J, Song H, et al. (2022) A novel anti-cancer therapy: CRISPR/Cas9 gene editing. Front. Pharmacol 13: 939090.
- Li H, Yang Y, Hong W, Huang M, Wu M, et al. (2020) Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal transduction and targeted therapy 5(1): 1.
- Kim H, Kim J-S (2014) A guide to genome engineering with programmable nucleases. Nature Reviews Genetics 15(5): 321-334.
- Akram F, Sahreen S, Aamir F, Haq IU, Malik K, et al. (2023) An insight into modern targeted genome-editing technologies with a special focus on CRISPR/Cas9 and its applications. Mol Biotechnol 65(2): 227-242.
- Maeder ML, Gersbach CA (2016) Genome-editing technologies for gene and cell therapy. Molecular Therapy 24(3): 430-446.
- Hadipour K, Asadishad T, Mahya L, Mohammadhassan R, Rahbar A, et al. (2023) A Comparative Review on Genome Editing Approaches. 13: 567.
- Liu Z, Shi M, Ren Y, Xu H, Weng S, et al. (2023) Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy. Mol Cancer 22(1):
- Chen B, Liu C, Long H, Bai G, Zhu Y, et al. (2023) N6‑methyladenosine‑induced long non‑coding RNA PVT1 regulates the miR‑27b‑3p/BLM axis to promote prostate cancer progression. Int J Oncol 62(1): 16.
- González Castro N, Bjelic J, Malhotra G, Huang C, Alsaffar SH (2021) Comparison of the feasibility, efficiency, and safety of genome editing technologies. Int J Mol Sci 22(19): 10355.
- Lu C, Kuang J, Shao T, Xie S, Li M, et al. (2022) Prime editing: an all-rounder for genome editing. Int J Mol Sci 23(17): 9862.
- Guo T, Feng YL, Xiao JJ, Liu Q, Sun XN, et al. (2018) Harnessing accurate non-homologous end joining for efficient precise deletion in CRISPR/Cas9-mediated genome editing. Genome Biol 19(1): 1-20.
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169(12): 5429-5433.
- Ishino Y, Krupovic M, Forterre P (2018) History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J Bacteriol 200(7): e00580-00517.
- Gostimskaya I (2022) CRISPR-Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing. Biochemistry (Mosc) 87(8): 777-788.
- Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, et al. (2020) Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 18(2): 67-83.
- Ledford H, Callaway E (2020) Pioneers of CRISPR gene editing win chemistry Nobel. Nature 586(7829): 346-347.
- Westermann L, Neubauer B, Köttgen M (2021) Nobel Prize 2020 in Chemistry honors CRISPR: a tool for rewriting the code of life. Pflügers Archiv-European Journal of Physiology 473(1): 1-2.
- Lu B, Guo Z, Zhong K, Osire T, Sun Y, et al. (2023) State of the art in CRISPR/Cas system-based signal conversion and amplification applied in the field of food analysis. Trends in Food Science & Technology 135(6299): 174-189.
- Van Der Oost J, Westra ER, Jackson RN, Wiedenheft B (2014) Unravelling the structural and mechanistic basis of CRISPR–Cas systems. Nature Reviews Microbiology 12(7): 479-492.
- Terns MP, Terns RM (2011): CRISPR-based adaptive immune systems. Curr Opin Microbiol 14(3): 321-327.
- Hille F, Charpentier E (2016) CRISPR-Cas: biology, mechanisms and relevance. Philos Trans R Soc Lond B: Biol Sci 371(1707): 20150496.
- Bhaya D, Davison M, Barrangou R (2011) CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Ann Rev Genet 45: 273-297.
- Uddin F, Rudin CM, Sen T (2020) CRISPR gene therapy: applications, limitations, and implications for the future. Front Oncol 10: 1387.
- Cai P, Gao J, Zhou Y (2019) CRISPR-mediated genome editing in non-conventional yeasts for biotechnological applications. Microb Cell Fact 18: 1-12.
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, et al. (2012) A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096): 816-821.
- Faure G, Shmakov SA, Makarova KS, Wolf YI, Crawley AB, et al. (2019) Comparative genomics and evolution of trans-activating RNAs in Class 2 CRISPR-Cas systems. RNA Biol 16(4): 435-448.
- Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, et al. (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156(5): 935-949.
- Wong N, Liu W, Wang X (2015) WU-CRISPR: characteristics of functional guide RNAs for the CRISPR/Cas9 system. Genome Biol 16(1): 1-8.
- Xue C, Greene EC (2021) DNA repair pathway choices in CRISPR-Cas9-mediated genome editing. Trends Genet 37(7): 639-656.
- Allen D, Rosenberg M, Hendel A (2021) Using synthetically engineered guide RNAs to enhance CRISPR genome editing systems in mammalian cells. Front Genome Ed 2: 617910.
- Konstantakos V, Nentidis A, Krithara A, Paliouras G (2022) CRISPR–Cas9 gRNA efficiency prediction: an overview of predictive tools and the role of deep learning. Nucleic Acids Res 50(7): 3616-3637.
- Pourcel C, Salvignol G, Vergnaud G (2005) CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151(3): 653-663.
- Mojica FJ, Díez Villaseñor Cs, García-Martínez J, Soria E (2005) Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 60: 174-182.
- Deveau H, Barrangou R, Garneau JE, Labonté J, Fremaux C, et al. (2008) Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol 190(4): 1390-1400.
- Montecillo JAV, Chu LL, Bae H (2020) CRISPR-Cas9 system for plant genome editing: Current approaches and emerging developments. Agronomy 10(7): 1033.
- Gleditzsch D, Pausch P, Müller-Esparza H, Özcan A, Guo X, et al. (2019) PAM identification by CRISPR-Cas effector complexes: diversified mechanisms and structures. RNA Biology 16(4): 504-517.
- Gürel F, Zhang Y, Sretenovic S, Qi Y (2020) CRISPR-Cas nucleases and base editors for plant genome editing. aBIOTECH 1:74-87.
- Zhang F, Wen Y, Guo X (2014) CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet 23(R1): R40-R46.
- Karvelis T, Gasiunas G, Miksys A, Barrangou R, Horvath P, et al. (2013) crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus. RNA Biol 10(5): 841-851.
- Wright AV, Nuñez JK, Doudna JA (2016) Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell 164(1-2): 29-44.
- Le Cong F (2013) Ran/Cox. David/Lin, Shuailiang/Barretto, Robert/Habib, Naomi/Hsu, Patrick D/Wu, Xuebing/Jiang, Wenyan/Marraffini, Luciano A/Zhang Feng: 819-823.
- Mali P, Esvelt KM, Church GM (2013) Cas9 as a versatile tool for engineering biology. Nat Methods 10(10): 957-963.
- Lechler MB (2021) CRISPR/Cas9-mediated genome engineering of the SMARCB1 gene locus.
- Ferreira da Silva J, Salic S, Wiedner M, Datlinger P, Essletzbichler P, et al. (2019) Genome-scale CRISPR screens are efficient in non-homologous end-joining deficient cells. Sci Rep 9(1): 1-10.
- Javed MR, Sadaf M, Ahmed T, Jamil A, Nawaz M, et al. (2018) CRISPR-Cas system: history and prospects as a genome editing tool in microorganisms. Curr Microbiol 75: 1675-1683.
- Cady KC, Bondy Denomy J, Heussler GE, Davidson AR, O'Toole GA (2012) The CRISPR/Cas adaptive immune system of Pseudomonas aeruginosa mediates resistance to naturally occurring and engineered phages. J Bacteriol 194(21): 5728-5738.
- Shabbir MAB, Hao H, Shabbir MZ, Hussain HI, Iqbal Z, et al. (2016) Survival and evolution of CRiSPR–Cas system in prokaryotes and its applications. Front Immunol 7: 375.
- Hryhorowicz M, Lipiński D, Zeyland J, Słomski R (2017) CRISPR/Cas9 immune system as a tool for genome engineering. Arch Immunol Ther Exp 65: 233-240.
- Li R, Zatloukalova P, Muller P, Gil-Mir M, Kote S, et al. (2020) The MDM2 ligand Nutlin-3 differentially alters expression of the immune blockade receptors PD-L1 and CD276. Cell Mol Biol Lett 25(1): 1-21.
- Shojaei Baghini S, Gardanova ZR, Abadi SAH, Zaman BA, İlhan A, et al. (2022) CRISPR/Cas9 application in cancer therapy: a pioneering genome editing tool. Cell Mol Biol Lett 27(1): 1-37.
- Jo N, Sogabe Y, Yamada Y, Ukai T, Kagawa H, et al. (2019) Platforms of in vivo genome editing with inducible Cas9 for advanced cancer modeling. Cancer Sci 110(3): 926-938.
- Rafii S, Tashkandi E, Bukhari N, Al-Shamsi HO (2022) Current status of CRISPR/Cas9 application in clinical cancer research: opportunities and challenges. Cancers 14(4): 947.
- Stefanoudakis D, Kathuria-Prakash N, Sun AW, Abel M, Drolen CE, et al. (2023) The Potential Revolution of Cancer Treatment with CRISPR Technology. Cancers 15(6): 1813.
- Ottaviano G, Georgiadis C, Gkazi SA, Syed F, Zhan H, et al. (2022) Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for 31 treatment of children with refractory B cell leukemia. Sci Transl Med 14(668): eabq3010.
- Rezalotfi A, Fritz L, Förster R, Bošnjak B (2022) Challenges of CRISPR-Based gene editing in primary T cells. Int J Mol Sci 23(3): 1689.
- Xiang H, Ahmad A, Fu Y (2023) United Kingdom, Department of Histopathology, Royal National Orthopaedic Hospital, London, United Kingdom. Therapeutic Gene Correction Strategies Based on CRISPR Systems or Other Engineered Site-specific Nucleases: 74.
- Qasim W (2023) Genome-edited allogeneic donor “universal” chimeric antigen receptor T cells. Blood, The Journal of the American Society of Hematology 141(8): 835-845.
- Varadarajan I, Pierce E, Scheuing L, Morris A, El Chaer F, et al. (2023) Post-Hematopoietic Cell Transplantation Relapsed Acute Lymphoblastic Leukemia: Current Challenges and Future Directions. Onco Targets Ther 16: 1-16.
- Greenbaum U, Mahadeo KM, Kebriaei P, Shpall EJ, Saini NY (2020) Chimeric antigen receptor T-cells in B-acute lymphoblastic leukemia: state of the art and future directions. Front Oncol 10: 1594.
- von Einem JC, Guenther C, Volk HD, G rütz G, Hirsch D, et al. (2019) Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT‐ME‐1 trial. Int J Cancer 145(6): 1538-1546.
- Karssli M (2022) CRISPR technology in human medicine: advantages and disadvantages over traditional treatments. Hochschule Rhein-Waal.
- Cornu TI, Mussolino C, Müller MC, Wehr C, Kern WV, et al. (2021) HIV gene therapy: an update. Hum Gene Ther 32(1-2): 52-65.
- Mirza Z, Karim S (2019) Advancements in CRISPR/Cas9 technology-focusing on cancer therapeutics and beyond. In: Semin Cell Dev Biol 96: 13-21.
- Yang Y (2017) PD-1 knockout EBV-CTLs for advanced stage Epstein-Barr virus (EBV) associated malignancies.
- Tischer-Zimmermann S, Bonifacius A, Santamorena MM, Mausberg P, Stoll S, et al. (2023) Reinforcement of cell-32 mediated immunity driven by tumor-associated Epstein-Barr virus (EBV)-specific T cells during targeted B-cell therapy with rituximab. Front Immunol 14: 878953.
- Nair J, Nair A, Veerappan S, Sen D (2020) Translatable gene therapy for lung cancer using Crispr CAS9-an exploratory review. Cancer Gene Ther 27(3-4): 116-124.
- Werner Sunderland M, Peggs KS (2018) Successful translation and future prospects of TALEN editing for leukemia patients. Expert Opin Biol Ther 18(7): 725-726.
- Ahmad MK, Theva Das K, Abdul Razak SR (2023) CRISPR/Cas9 technology: A promising gene-editing tool for the treatment of cancers. Iranian Journal of Blood and Cancer 15(1): 60-70.
- Ugalde L, Fañanas S, Torres R, Quintana-Bustamante O, Río P (2023) Clustered regularly interspaced short palindromic repeats/Cas9-mediated gene editing. A promising strategy in hematological disorders Cytotherapy.
- Ghaffari S, Khalili N, Rezaei N (2021) CRISPR/Cas9 revitalizes adoptive T-cell therapy for cancer immunotherapy. J Exp Clin Cancer Res 40(1): 269.
- Pavlovic K, Tristán-Manzano M, Maldonado-Pérez N, Cortijo-Gutierrez M, Sánchez-Hernández S, et al. (2020) Using gene editing approaches to fine-tune the immune system. Front Immunol 11: 570672.
- Ghosh D, Venkataramani P, Nandi S, Bhattacharjee S (2019) CRISPR–Cas9 a boon or bane: the bumpy road ahead to cancer therapeutics. Cancer Cell Int 19: 1-10.
- Bhatia S, Yadav SK (2023) CRISPR-Cas for genome editing: Classification, mechanism, designing and applications. Int J Biol Macromol 238: 124054.
- Khanam A, Kottilil S (2023) New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter? Int J Mol Sci 24(1): 437.
- Liu X, Li G, Liu Y, Zhou F, Huang X, et al. (2023) Advances in CRISPR/Cas gene therapy for inborn errors of immunity. Front Immunol 14: 1111777.
- Chakraborty E, Sarkar D (2022) Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 14(11): 2798.
- Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, et al. (2021) CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med 27(10): 1797-1805.
- Xie Y, Li X, Zeng H, Boucetta H, Wu J, et al. (2023) CAR-T cells for cancer immunotherapy. Chinese Chemical Letters: 108202.
- Albanyan O, Chavez J, Munoz J (2022) The role of CAR-T cell therapy as second line in diffuse large B-cell lymphoma. Ther Adv Hematol 13: 20406207221141511.
- McGuirk JP, Tam CS, Kröger N, Riedell PA, Murthy HS, et al. (2022) CTX110 Allogeneic CRISPR-Cas9-Engineered CAR T Cells in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results from the Phase 1 Dose Escalation Carbon Study. Blood 140(Suppl 1): 10303-10306.
- Wang Z, Chen M, Zhang Y, Liu Y, Yang Q, et al. (2020) Phase I study of CRISPR-engineered CAR-T cells with PD-1 inactivation in treating mesothelin-positive solid tumors. American Society of Clinical Oncology 38(15_suppl): 3038-3038.
- McGowan E, Lin Q, Ma G, Yin H, Chen S, et al. (2020) PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed Pharmacother 121: 109625.
- Schepisi G, Conteduca V, Casadei C, Gurioli G, Rossi L, et al. (2020) Potential application of chimeric antigen receptor (CAR)-T cell therapy in renal cell tumors. Front Oncol 10: 565857.
- Kim TJ, Lee YH, Koo KC (2022) Current and future perspectives on CAR-T cell therapy for renal cell carcinoma: A comprehensive review. Investig Clin Urol 63(5): 486-498.
- Rodríguez-Otero P, Prósper F, Alfonso A, Paiva B, Miguel JFS (2020) CAR T-cells in multiple myeloma are ready for prime time. J Clinical Med 9(11): 3577.
- Bruno B, Wäsch R, Engelhardt M, Gay F, Giaccone L, et al. (2021) European Myeloma Network perspective on CAR T-Cell therapies for multiple myeloma. Haematologica 106(8): 2054-2065.
- Wang Z, Li N, Feng K, Chen M, Zhang Y, et al. (2021) Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol 18(9): 2188-2198.
- Abreu TR, Fonseca NA, Gonçalves N, Moreira JN (2020) Current challenges and emerging opportunities of CAR-T cell therapies. J Control Release 319: 246-261.
- Naeem M, Hazafa A, Bano N, Ali R, Farooq M, et al. (2023) Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in cancer immunotherapy. Life Sci 316: 121409.
- Hiltensperger M, Krackhardt AM (2023) Current and future concepts for the generation and application of genetically engineered CAR-T and TCR-T cells. Front Immunol 14: 1121030.
- van Amerongen RA, Tuit S, Wouters AK, van de Meent M, Siekman SL, et al. (2023) PRAME and CTCFL-reactive TCRs for the treatment of ovarian cancer. Front Immunol 14:1121973.
- Lanza F, Rondoni M, Zannetti BA (2023) New Horizons in Immunology and Immunotherapy of Acute Leukemias and Related Disorders. Cancers 15(9): 2422.
- Jacinto FV, Link W, Ferreira BI (2020) CRISPR/Cas9‐mediated genome editing: From basic research to translational medicine. J Cell Mol Med 24(7): 3766-3778.
- Nejatisafa A-A, Faccio F, Nalini R (2020) Psychological aspects of pregnancy and lactation in patients with breast cancer: Adv Exp Med Biol 1252: 199-207.
- Sayin VI, Papagiannakopoulos T (2017) Application of CRISPR-mediated genome engineering in cancer research. Cancer Lett 387: 10-17.
- Mohammad Rafiei F, Safdarian E, Adel B, Vandchali NR, Navashenaq JG, et al. (2023) CRISPR: A Promising Tool for Cancer Therapy. Curr Mol Med 23(8): 748-761.
- Lok BH, Gardner EE, Schneeberger VE, Teicher BA, Riaz N, et al. (2017) PARP Inhibitor Activity Correlates with SLFN11 Expression and Demonstrates Synergy with 35 Temozolomide in Small Cell Lung CancerSLFN11 Predicts PARP Inhibitor Response in SCLC. Clin Cancer Res 23(2): 523-535.
- Santoni Rugiu E, Melchior LC, Urbanska EM, Jakobsen JN, de Stricker K, et al. (2019) Intrinsic resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: differences and similarities with acquired resistance. Cancers 11(7): 923.
- McFadden DG, Papagiannakopoulos T, Taylor Weiner A, Stewart C, Carter SL, et al. (2014) Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 156(6): 1298-1311.
- Huang J, Chen M, Xu ES, Luo L, Ma Y, et al. (2019) Genome-wide cRiSpR Screen to identify Genes that Suppress transformation in the presence of endogenous KrasG12D. Sci Rep 9(1): 17220.
- Ouyang Q, Liu Y, Tan J, Li J, Yang D, et al. (2019) Loss of ZNF587B and SULF1 contributed to cisplatin resistance in ovarian cancer cell lines based on Genome-scale CRISPR/Cas9 screening. Am J Cancer Res 9(5): 988.
- Zhang L, Yang Y, Chai L, Bu H, Yang Y, et al. (2020) FRK plays an oncogenic role in non‐small cell lung cancer by enhancing the stemness phenotype via induction of metabolic reprogramming. Int J Cancer 146(1): 208-222.
- Cheong T, Blasco R, Chiarle R (2018) Chromosome Translocation. Advances in Experimental Medicine and Biology.
- Khalaf K, Janowicz K, Dyszkiewicz Konwińska M, Hutchings G, Dompe C, et al. (2020) CRISPR/Cas9 in cancer immunotherapy: animal models and human clinical trials. Genes 11(8): 921.
- Zhao KR, Wang L, Liu PF, Hang XM, Wang HY, et al. (2021) A signal-switchable electrochemiluminescence biosensor based on the integration of spherical nucleic acid and CRISPR/Cas12a for multiplex detection of HIV/HPV DNAs. Sensors and Actuators B: Chemical 346(2019): 130485.
- Kennedy EM, Cullen BR (2015) Bacterial CRISPR/Cas DNA endonucleases: A revolutionary technology that could dramatically impact viral research and treatment. Virology 479: 213-220.
- De Paoli P, Carbone A (2013) Carcinogenic viruses and solid cancers without sufficient evidence of causal association. Int J Cancer 133(7): 1517-1529.
- Donà S, Borsetto D, Fussey J, Biscaro V, Vian E, et al. (2019) Association between hepatitis C and B viruses and head and neck squamous cell carcinoma. J Clin Virol 121: 104209.
- Kostyushev D, Kostyusheva A, Brezgin S, Zarifyan D, Utkina A, et al. (2019) Suppressing the NHEJ pathway by DNA-PKcs inhibitor NU7026 prevents degradation of HBV cccDNA cleaved by CRISPR/Cas9. Sci Rep 9(1): 1847.
- Hu Z, Yu L, Zhu D, Ding W, Wang X, et al. (2014) Disruption of HPV16-E7 by CRISPR/Cas system induces apoptosis and growth inhibition in HPV16 positive human cervical cancer cells. Biomed Res Int 2014: 612823.
- Akasu M, Shimada S, Kabashima A, Akiyama Y, Shimokawa M, et al. (2021) Intrinsic activation of β-catenin signaling by CRISPR/Cas9-mediated exon skipping contributes to immune evasion in hepatocellular carcinoma. Scientific reports 11(1): 16732.
- Srour N, Villarreal OD, Hardikar S, Yu Z, Preston S, et al. (2022) PRMT7 ablation stimulates anti-tumor immunity and sensitizes melanoma to immune checkpoint blockade. Cell Reports 38(13):110582.
- Kang K, Song Y, Kim I, Kim T-J (2022) Therapeutic applications of the CRISPR-Cas system. Bioengineering 9(9):477.
- Bhat ZR, Gahlawat A, Kumar N, Sharma N, Tikoo K, et al. (2023) Target Validation and Structure-Based Virtual Screening to Discover Potential Lead Molecules against the oncogenic NSD1 histone methyltransferase. 11(1): 21.
- Zhang S, Zhang F, Chen Q, Wan C, Xiong J, et al. (2019) CRISPR/Cas9-mediated knockout of NSD1 suppresses the hepatocellular carcinoma development via the NSD1/H3/Wnt10b signaling pathway. J Exp Clin Cancer Res 38(1): 467.
- Pan Q, Xie Y, Zhang Y, Guo X, Liu M (2023) EGFR core fucosylation, induced by enveloped viruses, promotes TRIM40-mediated-RIG-I ubiquitination and suppresses interferon-I antiviral defenses.
- Zare K, Shademan M, Ghahramani Seno MM, Dehghani H (2018) CRISPR/Cas9 knockout strategies to ablate CCAT1 lncRNA gene in cancer cells. Biological procedures online 20: 1-12.
- Zhang J, Hu K, Yang Y-q, Wang Y, Zheng Y-f, et al. (2020) LIN28B-AS1-IGF2BP1 binding promotes hepatocellular carcinoma cell progression. Cell Death & Disease 11(9): 741.
- Suzuki K, Masuike Y, Mizuno R, Sachdeva UM, Chatterji P, et al. (2021) LIN28B induces a differentiation program through CDX2 in colon cancer. JCI insight 6(9): e140382.
- Oura K, Morishita A, Hamaya S, Fujita K, Masaki T (2023) The roles of epigenetic regulation and the tumor microenvironment in the mechanism of resistance to systemic therapy in hepatocellular carcinoma. Int J Mol Sci 24(3): 2805.
- Yang Y, Yan Y, Yin J, Tang N, Wang K, et al. (2023) O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N6-methyladenosine-dependent manner. Signal Transduct Target Ther 8(1): 63.
- Song J, Zhang X, Ge Q, Yuan C, Chu L, et al. (2018) CRISPR/Cas9‐mediated knockout of HBsAg inhibits proliferation and tumorigenicity of HBV‐positive hepatocellular carcinoma cells. J Cell Biochem 19(10): 8419-8431.
- Xie J-y, Wei J-x, Lv L-h, Han Q-f, Yang W-b, et al. (2020) Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal 18(1): 46.
- Ren A, Gan Q, Han W, Gong D, Cai J, et al. (2022) Endothelial GATA5 positively regulates angiogenesis via cathepsin S-mediated Angpt2/Flk1 and MMP2/9 signaling pathways. Biochem Biophys Res Commun 609: 111-118.
- Li W, Xu L, Cao J, Ge J, Liu X, et al. (2023) DACH1 regulates macrophage activation and tumour progression in hypopharyngeal squamous cell carcinoma. Immunology 170: 253-269.
- Cantonero C, Sánchez-Collado J, Salido GM, Rosado JA, Redondo PC, et al. (2020) Arachidonic acid attenuates cell proliferation, migration and viability by a mechanism independent on calcium entry. International journal of molecular sciences 21(9): 3315.
- Ghobashi AH, Vuong TT, Kimani JW, O’Hagan HM (2023) Activation of AKT induces EZH2-mediated β-catenin trimethylation in colorectal cancer. bioRxiv 4: 2023.01.31.526429.
- Sanati M, Afshari AR, Ahmadi SS, Moallem SA, Sahebkar A (2023) Modulation of the ubiquitin‐proteasome system by phytochemicals: Therapeutic implications in malignancies with an emphasis on brain tumors. Biofactors 49(4): 782-819.
- Li X, Chen X, Gong S, Zhao J, Yao C, et al. (2023) Platelets promote CRC by activating the C5a/C5aR1 axis via PSGL-1/JNK/STAT1 signaling in tumor-associated macrophages. Theranostics 13(6): 2040-2056.
- Xu Y-j, Zeng K, Ren Y, Mao C-y, Ye Y-h, et al. (2023) Inhibition of USP10 induces myeloma cell apoptosis by promoting cyclin D3 degradation. Acta Pharmacol Sin 44(9): 1920-1931.
- Xu Z, Wu Y, Yang M, Wei H, Pu J (2023) CBX2-mediated suppression of SIAH2 triggers WNK1 accumulations to promote glycolysis in hepatocellular carcinoma. Exp Cell Res 426(1): 113513.
- Elballal MS, Sallam A-AM, Elesawy AE, Elrebehy MA, Elazazy O, et al. (2023) miRNAs as potential game-changers in renal cell carcinoma: Future Clinical and Medicinal Uses. Pathology-Research and Practice 245: 154439.
- Sharma K, Sharma S, Kanwar JR (2023) MicroRNA Signatures of Tumor Hypoxia. In: Hypoxia in Cancer: Significance and Impact on Cancer Therapy 139-159.
- Chen F, Li Y, Aye L, Wu Y, Dong L, et al. (2023) FUT8 is regulated by miR‐122‐5p and promotes malignancies in intrahepatic cholangiocarcinoma via PI3K/AKT signaling. Cell Oncol 46(1): 79-91.
- Singhal SS, Garg R, Mohanty A, Garg P, Soldi R, et al. (2023) Recent Advancement in Breast Cancer Research: Insights from Model Organisms—Mouse Models to Zebrafish. Cancers 15(11): 2961.
- Liu H, Wen T, Zhou Y, Fan X, Du T, et al. (2019) DCLK1 plays a metastatic-promoting role in human breast cancer cells. BioMed Res Int 2019: 1061979.
- Ge Y, Liu H, Zhang Y, Liu J, Yan R, et al. (2022) Inhibition of DCLK1 kinase reverses epithelial-mesenchymal transition and restores T-cell activity in pancreatic ductal adenocarcinoma. Translational Oncology 17: 101317.
- Hariprabu KNG, Sathya M, Vimalraj S (2021) CRISPR/Cas9 in cancer therapy: A review with a special focus on tumor angiogenesis. Int J Biol Macromol 192: 913-930.
- Vesuna F, Penet M-F, Mori N, Bhujwalla ZM, Raman V (2023) Twist alters the breast tumor microenvironment via choline kinase to facilitate an aggressive phenotype. Molecular and Cellular Biochemistry 478(4): 939-948.
- Rezaee M, Mohammadi F, Keshavarzmotamed A, Yahyazadeh S, Vakili O, et al. (2023) The landscape of exosomal non-coding RNAs in breast cancer drug resistance, focusing on underlying molecular mechanisms. Front Pharmacol 14: 1152672.
- Nie Z, Gao Y, Chen M, Peng Y, Guo N, et al. (2023) Genome-Wide Screening Identifies Gene AKR1C1 Critical for Resistance to Pirarubicin in Bladder Cancer. Cancers 15(9): 2487.
- Ji J, Jin D, Xu M, Jiao Y, Wu Y, et al. (2022) AKR1B1 promotes pancreatic cancer metastasis by regulating lysosome-guided exosome secretion. Nano Research 15(6): 5279-5294.
- Grunblatt E, Wu N, Zhang H, Liu X, Norton JP, et al. (2020) MYCN drives chemoresistance in small cell lung cancer while USP7 inhibition can restore chemosensitivity. Genes Dev 34(17-18): 1210-1226.
- Shin S-B, Kim C-H, Jang H-R, Yim H (2020) Combination of Inhibitors of USP7 and PLK1 has a Strong Synergism against Paclitaxel Resistance. Int J Mol Sci 21(22): 8629.
- Li J, Cheng D, Zhu M, Yu H, Pan Z, et al. (2019) OTUB2 stabilizes U2AF2 to promote the Warburg effect and tumorigenesis via the AKT/mTOR signaling pathway in non-small cell lung cancer. Theranostics 9(1): 179-195.
- Xu Y, Pan J, Lin Y, Wu Y, Chen Y, et al. (2023) Ceramide Synthase 1 Inhibits Brain Metastasis of Non-Small Cell Lung Cancer by Interacting with USP14 and Downregulating the PI3K/AKT/mTOR Signaling Pathway. Cancers 15(7): 1994.
- Wang Z, Kong J, Wu Y, Zhang J, Wang T, et al. (2018) PRMT5 determines the sensitivity to chemotherapeutics by governing stemness in breast cancer. Breast Cancer Res Treat 168(2): 531-542.
- Yin S, Liu L, Brobbey C, Palanisamy V, Ball LE, et al. (2021) PRMT5-mediated arginine methylation activates AKT kinase to govern tumorigenesis. Nature communications 12(1): 3444.
- Zhang Y-Q, Pei J-H, Shi S-S, Guo X-s, Cui G-y, et al. (2019) CRISPR/Cas9-mediated knockout of the PDEF gene inhibits migration and invasion of human gastric cancer AGS cells. Biomed Pharmacother 111: 76-85.
- Chen H-H, Hao P-H, Zhang F-Y, Zhang T-N (2023) Non-coding RNAs in metabolic reprogramming of bone and soft tissue sarcoma: Fundamental mechanism and clinical implication. Biomed Pharmacother 160: 114346.
- Wang Y, Yang Z (2023) METTL3 relieved the injury of SH‐SY5Y cells treated with lipopolysaccharide and exposed to sevoflurane through regulating the m6A levels of Sox2. Brain and Behav 13(5): e2936.
- Zhao S, Song P, Zhou G, Zhang D, Hu Y (2023) METTL3 promotes the malignancy of non-small cell lung cancer by N6-methyladenosine modifying SFRP2. Cancer Gene Therapy 30(8):1094-1104.
- Sivakumar S, Lieber S, Librizzi D, Finkernagel F, Roth K, et al. (2023) Basal cell adhesion molecule promotes metastasis‐associated processes in ovarian cancer. Clinical and Translational Medicine 13(1): e1176.
- Liu X, Sun J, Gu J, Weng L, Wang X, et al. (2023) Effective drug and shRNA delivery for synergistic treatment of triple-negative breast cancer by sequentially targeting tumor hypoxia. Chemical Engineering Journal 470: 144271.
- Yasunaga K, Ito T, Miki M, Ueda K, Fujiyama T, et al. (2018) Using CRISPR/Cas9 to knock out amylase in acinar cells decreases pancreatitis-induced autophagy. Biomed Res Int 2018: 8719397.
- İzmirli M (2023) The HANDBOOK on CANCER. Holistence Publications.
- Alnefaie A, Albogami S, Asiri Y, Al-Sanea MM, Althobaiti H (2022) Chimeric Antigen Receptor T-Cells: An Overview of Concepts, Applications, Limitations, and Proposed Solutions. Front Bioeng Biotechnol 10: 797440.
- Alborzinia H, Flórez AF, Kreth S, Brückner LM, Yildiz U, et al. (2022) MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nature cancer 3(4): 471-485.
- De Rosa P, Severi F, Zadran SK, Russo M, Aloisi S, et al. (2023) MYCN Amplification, along with Wild-Type RB1 Expression, Enhances CDK4/6 Inhibitors’ Efficacy in Neuroblastoma Cells. Int J Mol Sci 24(6): 5408.
- Liu B, Zhang J, Meng X, Xie SM, Guo M, et al. (2023) HDAC6-G3BP2 promotes lysosomal-TSC2 and suppresses mTORC1 under ETV4 targeting-induced low-lactate stress in non-small cell lung cancer. Oncogene 42(15): 1181-1195.
- Cosi I, Moccia A, Pescucci C, Munagala U, Di Giorgio S, et al. (2023) Identification and characterization of novel ETV4 splice variants in prostate cancer. Scientific Reports 13(1): 5267.
- Hao Z, Huajun S, Zhen G, Yu X, Qian L, et al. (2023) AQP8 promotes glioma proliferation and growth, possibly through the ROS/PTEN/AKT signaling pathway. BMC cancer 23(1): 516.
- Guo L, Dong Z, Zhang X, Yang Y, Hu X, et al. (2023) Morusinol extracted from Morus alba induces cell cycle arrest and apoptosis via inhibition of DNA damage response in melanoma by CHK1 degradation through the ubiquitin-proteasome pathway. Phytomedicine 114: 154765.
- Li Y, Yu P, Long J, Tang L, Zhang X, et al. (2022) A novel ribosomal protein S6 kinase 2 inhibitor attenuates the malignant phenotype of cutaneous malignant melanoma cells by inducing cell cycle arrest and apoptosis. Bioengineered 13(5): 13555-13570.
- Farheen J, Hosmane NS, Zhao R, Zhao Q, Iqbal MZ, et al. (2022) Nanomaterial-assisted CRISPR gene-engineering–A hallmark for triple-negative breast cancer therapeutics advancement. Materials Today Bio 2022: 100450.
- Zhao Q, Qian Q, Cao D, Yang J, Gui T, et al. (2018) Role of BMI1 in epithelial ovarian cancer: investigated via the CRISPR/Cas9 system and RNA sequencing. J Ovarian Res 11(1): 31.
- Li F, Zheng Z, Wei C, Zhang H, Li D, et al. (2023) Regulation of cisplatin resistance in bladder cancer by epigenetic mechanisms. Drug Resist Updat 100938.
- Zhang Y, Tang J, Wang C, Zhang Q, Zeng A, et al. (2023) Autophagy-related lncRNAs in tumor progression and drug resistance: A double-edged sword. Genes Dis 11(1): 367-381.
- Zhang Z-L, Rong R, Ren X-L, Xu L-W, Lian W-J, et al. (2023) CX-5461-inspired monofunctional platinum RNA polymerase I selective inhibitors with selective lethality in BRCA1-deficient cancer cells. Inorganic Chemistry Frontiers.
- Yang L, Fan Q, Wang J, Yang X, Yuan J, Li Y, et al. (2023) TRPS1 regulates the opposite effect of progesterone via RANKL in endometrial carcinoma and breast carcinoma. Cell Death Discovery 9(1): 185.
- Wang Y, Liu M, Guo X (2023) LINC00963-FOSB-mediated transcription activation of UBE3C enhances radioresistance of breast cancer cells by inducing ubiquitination-dependent protein degradation of TP73. Journal of Translational Medicine 21(1): 1-17.
- Su B, Zhang L, Zhuang W, Zhang W, Chen X (2021) Knockout of Akt1/2 suppresses the metastasis of human prostate cancer cells CWR22rv1 in vitro and in vivo. Journal of Cellular and Molecular Medicine 25(3): 1546-1553.
- Mehta D, Stürchler A, Anjanappa RB, Zaidi SS-e-A, Hirsch-Hoffmann M, et al. (2019) Linking CRISPR-Cas9 interference in cassava to the evolution of editing-resistant geminiviruses. Genome Biol 20(1): 80.
- Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, et al. (2017) RNA targeting with CRISPR–Cas13. Nature 550(7675): 280-284.
- Aman R, Ali Z, Butt H, Mahas A, Aljedaani F, et al. (2018) RNA virus interference via CRISPR/Cas13a system in plants. Genome biology 19(1): 1.
- Barman HN, Sheng Z, Fiaz S, Zhong M, Wu Y, et al. (2019) Generation of a new thermo-sensitive genic male sterile rice line by targeted mutagenesis of TMS5 gene through CRISPR/Cas9 system. BMC Plant Biol 19(1): 109.
- Shen L, Hua Y, Fu Y, Li J, Liu Q, et al. (2017) Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice. Sci China Life Sci 60(5):506-515.
- Bao A, Burritt DJ, Chen H, Zhou X, Cao D, et al. (2019) The CRISPR/Cas9 system and its applications in crop genome editing. Crit Rev Biotechnol 39(3): 321-336.
- Fiaz S, Ahmad S, Noor MA, Wang X, Younas A, et al. (2019) Applications of the CRISPR/Cas9 system for rice grain quality improvement: perspectives and opportunities. Int J Mol Sci 20(4): 888.
- Oliva R, Ji C, Atienza-Grande G, Huguet-Tapia JC, Perez-Quintero A, et al. (2019) Broad-spectrum resistance to bacterial blight in rice using genome editing. Nature biotechnology 37(11): 1344-1350.
- Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, et al. (2020) CRISPR–Cas12-based detection of SARS-CoV-2. Nature biotechnology 38(7): 870-874.
- Abbott TR, Dhamdhere G, Liu Y, Lin X, Goudy L, et al. (2020) Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell 181(4): 865-876.
- Nasser TA, AbdElhamid AS, Teleb M, Khattab SN, Bekhit AA, et al. (2022) Chapter 15 - Concluding remarks and future perspective of combination drug delivery systems. Combination Drug Delivery Approach as an Effective Therapy for Various Diseases. 2022: 353-396.
-
Kainat Ramzan*, Imran Haider, Maliha Sarfraz, Ayesha Aslam, Noor Zada khan, Sonia, Sabahat Tanveer, Saira Ramzan and Hayat Ullah. A Pioneering Genome Editing Tool with Crispr-CAS9 Approach for Application in Cancer Therapy: Future Perspectives and Challenges. Arch Biomed Eng & Biotechnol. 7(5): 2024. ABEB.MS.ID.000673.
-
Cancer immunotherapy; WHO; FDA; EMA; CART; Agrobacterium
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






