 Research Article
Research Article
Non-Invasive Oscillating Device Use as Chest Physiotherapy in Critical Care, Comparing Conventional Chest Physiotherapy A Pilot Crossover Randomized Controlled Trial
Marika Matsumoto MD1*, Atsushi Kawaguchi MD PhD1,2, Kosar Khwaja MD MBA3, Matthew P Cheng MD4, Todd Lee MD MPH3 and Philippe Jouvet MD MBA PhD2
1Division of Pediatric Critical Care, Department of Pediatrics, St Marianna University School of Medicine, Kawasaki, Kanagawa, Japan
2Department of Pediatrics, University of Montreal, CHU Sainte-Justine, Montreal, Quebec, Canada
3Departments of Surgery and Critical Care Medicine, McGill University, Montreal, Canada
4Division of Infectious Diseases and Medical Microbiology, McGill University Health Centre, Montréal, Quebec, Canada
Atsushi Kawaguchi, Division of Pediatric Critical Care, Department of Pediatrics, St. Marianna University School of Medicine. 2-16-1, Sugao, Miyamae, Kawasaki, 216-8511
Received Date: October 09, 2025; Published Date: October 22, 2025
Abstract
While airway-clearance devices are commonly used for chronic respiratory diseases, evidence supporting their use—especially in critically ill patients—is limited. We conducted a pilot study to assess the feasibility and procedure safety of a noninvasive chest wall oscillation device (NIOD) delivered by non-physiotherapist staff, compared with conventional chest physiotherapy (cCPT) performed by physiotherapists. Sixteen patients with COVID-19 were enrolled. The intervention completion rate was 100% (16/16) for NIOD and 93.8% (15/16) for cCPT. One planned cCPT session was withheld because of hemodynamic instability considered unrelated to the study interventions. No adverse events were attributed to either procedure. Changes from baseline in the SpO2/FiO2 (S/F) ratio and other vital signs were minimal and not clinically meaningful in either group at 10- and 30-minutes post-intervention. Work of breathing and neurological status also remained stable in most patients. In conclusion, NIOD was feasible to implement by non-physiotherapist staff and appeared safe in this pilot study, with no procedure-related adverse events. Short-term physiological effects were minimal for both interventions. These findings support proceeding to a subsequent definitive trial not limited to COVID-19 to evaluate the effectiveness and potential role of NIOD in critical care settings, including its use as an alternative to cCPT under physiotherapy staffing constraints.
Keywords:Pilot study; crossover-randomized-trial; chest physiotherapy; non-invasive oscillation device; critical care; COVID-19
Abbreviations:CPT: Chest Physiotherapy, CF: Cystic Fibrosis, COPD: Chronic Obstructive Pulmonary Disease, NIOD: Noninvasive chest wall Oscillation Device, cCPT: Conventional Chest Physiotherapy, ICU: Intensive Care Unit, SF: SpO2/FiO2 ratio, HR: Heart Rate, RR: Respiratory Rate, MAP: Mean Arterial Pressure, TV: Expiratory Tidal Volume, WOB: Work of Breathing, IQR: Interquartile Ranges
Introduction
Despite limited evidence for its effectiveness, chest physiotherapy (CPT) is widely used as an adjunct to respiratory care in critically ill patients [1,2]. Airway-clearance devices— including oscillatory modalities—are commonly employed in chronic conditions such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and neuromuscular disorders [3-5]. However, evidence supporting their use in critical care settings is scarce. Therefore, we conducted a pilot study to assess the feasibility of a noninvasive chest wall oscillation device (NIOD) delivered by non-physiotherapist staff for airway clearance in critically ill patients, compared with conventional chest physiotherapy (cCPT). The findings are intended to inform the design of a subsequent definitive trial to evaluate clinical effectiveness and the potential role of NIOD as an alternative to cCPT under physiotherapy staffing constraints, such as those experienced during the COVID-19 pandemic [6,7].
Material and Methods
Settings and Design
We conducted a pilot crossover randomized controlled trial in an adult Intensive Care Unit (ICU) of a Canadian university hospital.
Patients and Randomization
We recruited patients admitted to the ICU between September 2021 and April 2022 who were prescribed CPT for airway clearance. Patients were considered eligible only if CPT was expected to be performed for at least 24 hours after enrollment. Eligibility did not depend on respiratory support; patients receiving invasive mechanical ventilation, noninvasive ventilation, high-flow nasal cannula, or conventional oxygen therapy by mask or nasal cannula were included. For pragmatic reasons during the pandemic period, the cohort comprised patients with COVID-19, although the device is intended for use in critically ill patients irrespective of etiology. Following enrollment, participants were randomly assigned in a 1:1 ratio to one of two sequences: NIOD first or cCPT first. Randomization was performed by an independent research assistant using consecutively numbered, sealed, opaque envelopes. While the nature of the interventions precluded the blinding of patients and clinicians, outcome assessors were blinded to the treatment allocation.
Interventions
Participants received one session each of NIOD (Frequencer®, Dymedso, Montreal, Canada) and cCPT in randomized order, separated by a washout period of at least 3 hours. The cCPT session was delivered exclusively by physiotherapists. The specific cCPT techniques (e.g., percussion, vibration, compression) and session duration were determined based on the physiotherapist’s clinical judgment, reflecting institutional clinical practice. The NIOD session was administered by trained non-physiotherapist personnel (ICU nurses or respiratory therapists) following a standardized protocol. This protocol, supported by an instructional video, consisted of a 12-minute session targeting four chest wall regions (left anterior, right anterior, left lateral, and right lateral) for 3 minutes each. Device settings were fixed at a 50% intensity output and a frequency of 40 Hz. Throughout the study, all participants continued to receive standard intensive care, including airway suctioning, nebulized therapies, and ventilator adjustments, as directed by the attending intensivist.
Measurements and Analyses
The primary objectives of this pilot study were to assess feasibility and procedural safety. Feasibility was defined as the completion rate of scheduled intervention sessions. Safety was assessed by adverse events occurring during or up to 30 minutes post-intervention. Adverse events included device-related complications requiring clinical intervention, such as unplanned extubation. To inform the design of a future definitive trial, several exploratory outcomes were collected. The principal exploratory outcome was the change in the SpO2/FiO2 (S/F) ratio. Additional parameters included vital signs (Heart Rate (HR), Respiratory Rate (RR), Mean Arterial Pressure (MAP)), expiratory tidal volume (TV, in mechanically ventilated patients), work of breathing (WOB), and neurological status. The degree of WOB was classified as none, mild, moderate, or severe.
Neurological status was categorized as: 1) normal; 2) agitated when disturbed; 3) agitated without disturbance or depressed; and 4) markedly depressed or comatose. These assessments were performed by bedside nurses who were blinded to the intervention sequence. Measurements were obtained at three time points: immediately before the intervention and at 10- and 30-minutes post-intervention. Consistent with the pilot nature of this study, the analysis was primarily descriptive. Continuous variables were presented as medians with interquartile ranges (IQRs), and categorical variables as counts and percentages. For planning purposes, we fit exploratory linear mixed-effects models with a random intercept for patient to estimate between-intervention differences in change from baseline, adjusting for measurement time (before, 10, and 30 minutes after).
These analyses were underpowered and did not influence the study’s conclusions. A formal sample size calculation was not performed for this feasibility study. However, a target sample size of 25 participants was pre-specified in the registered protocol to ensure adequate data to guide the planning of a future definitive noninferiority trial. Analyses were performed using Stata (MP version 18.5, Stata Corp LLC, USA), and EZR (version 1.61, Jichi Medical University Saitama Medical Center, Japan). Informed consent was obtained from patients or their guardians. The study protocol was approved by the Ethical Committee at the University of Montreal and McGill University, Quebec, Canada. The study protocol details can be found in ClinicalTrials.gov (ID NCT03821389).
Results and Discussion
Patients Included
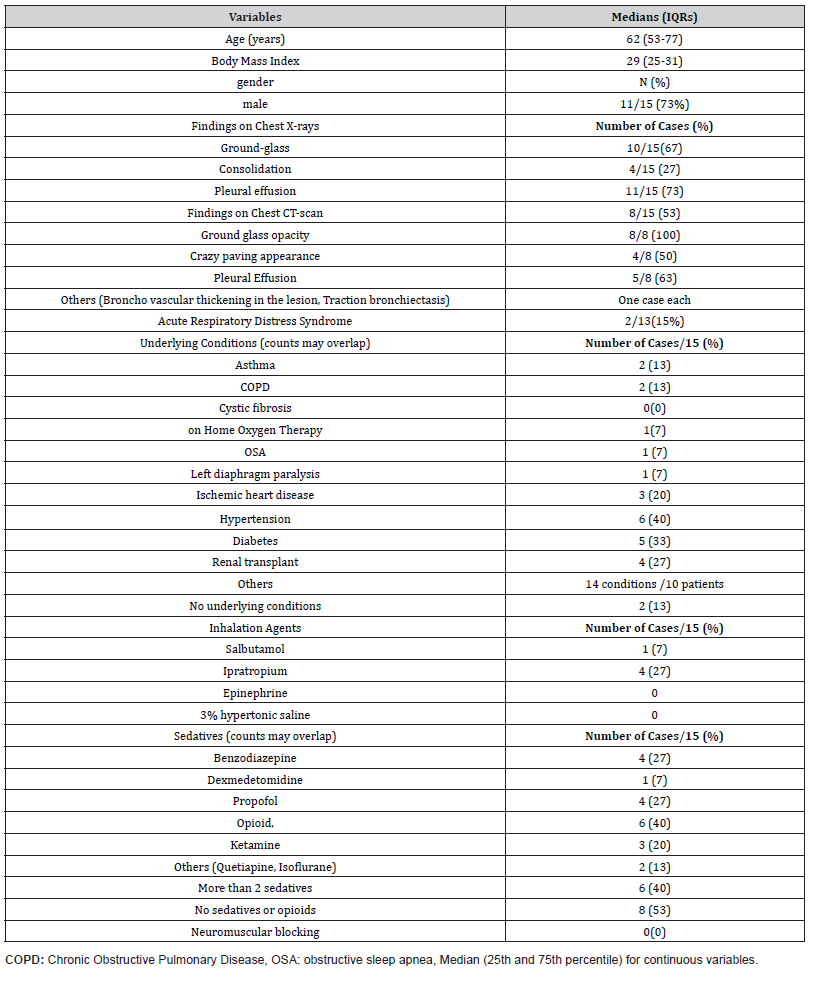
A total of 16 patients were enrolled. Baseline demographic and clinical characteristics were summarized in Table 1. The median age was 59 years (IQR, 52–77), and 75% were male. The median body mass index was 29 kg/m² (IQR, 25–31). All patients had abnormal findings on chest radiography (e.g., ground-glass opacities, consolidation, or pleural effusion). Baseline characteristics were comparable between the two intervention-sequence groups (Table 1).
Table 1: Baseline Demographic and Clinical Characteristics of the Cohort.
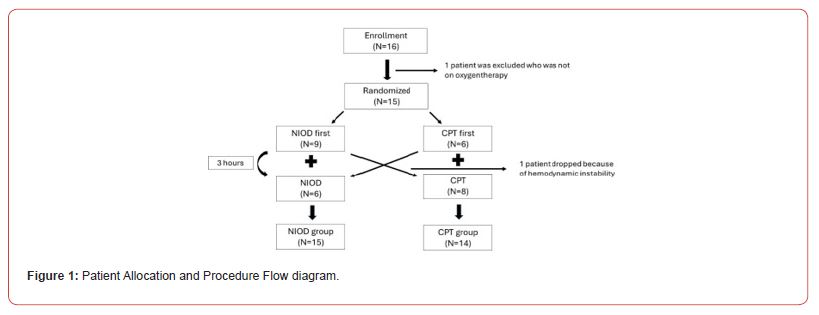
Feasibility Outcomes
The completion rate was 100% (16/16 planned sessions) for NIOD and 93.8% (15/16) for cCPT (Figure 1). One planned cCPT session was not administered because of hemodynamic instability that occurred prior to the session. The median interval between interventions was 4.0 hours (range, 3.2–5.2 hours).
Procedural Safety Outcomes
Two patients experienced notable clinical events, though neither was considered procedure-related. One patient did not receive the planned CPT due to hemodynamic instability that developed approximately 3 hours after NIOD; this was judged unrelated, given the time interval and the patient’s stable post-NIOD observations. Relative to baseline, MAP was +17 mmHg at 10 minutes and +16 mmHg at 30 minutes after the NIOD intervention, and HR was +7 bpm at 10 minutes and +1 bpm at 30 minutes after. Another patient with severe baseline hypoxemia required escalation from high-flow nasal cannula to CPAP after only transient improvement following NIOD; SpO2 increased from 84% pre-intervention to 89% at 10 minutes and 93% at 30 minutes after while receiving FiO2 1.0. Both events were considered consistent with part of the natural course of the patients’ critical illness.
Exploratory Physiological Outcomes
Table 2 summarizes median (IQR) values for each intervention at each time point, and Table 3 summarizes median (IQR) changes from baseline. Changes in physiological parameters at 10- and 30-minutes post-intervention were minimal for both interventions. For the primary exploratory outcome (S/F ratio), median changes from baseline were negligible in the NIOD sessions (−0.6 at 10 minutes; −1.5 at 30 minutes) and in the cCPT sessions (0 at both time points). In an exploratory linear mixed-effects model with a random intercept for patient, adjusted for measurement time, the estimated between-intervention difference in change in S/F (NIOD minus cCPT) was −3.28 (95% CI, −9.81 to 3.25), indicating no clinically meaningful difference.
Table 2: Results for Each Group at Each Time Point.
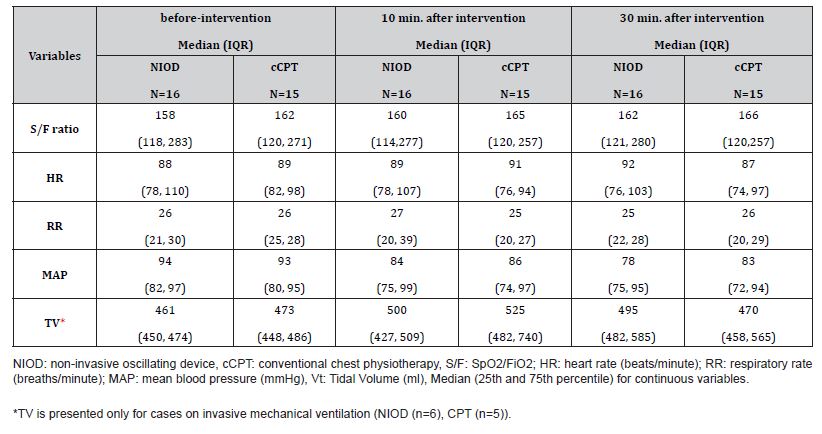
Table 3: Median [IQR] Change in Physiological Parameters from Baseline.
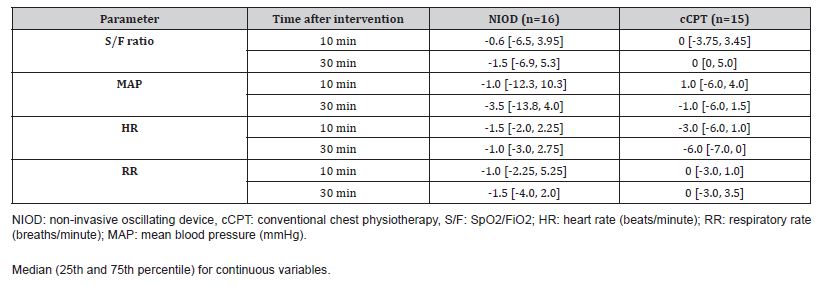
Similarly, no clinically meaningful changes were observed for other vital signs; median changes in MAP, HR, and RR were small across time points in both groups (Table 2). Work of breathing and neurological status remained stable for most participants. At baseline, WOB was none or mild in all sessions (NIOD: 13 none, 3 mild; cCPT: 11 none, 4 mild). Following NIOD, 15 of 16 sessions were unchanged and 1 improved (mild to none) at 30 minutes. Following the cCPT group, two patients had improved by 30 minutes, whereas one patient’s status changed from none to mild. The neurological status of all participants was stable and unchanged from baseline at all assessment time points for both interventions.
Discussions
In this single-center randomized crossover pilot study, we found that the NIOD delivered by non-physiotherapist staff was feasible (completion 100% for NIOD; 93.8% for conventional CPT) and appeared safe, with no procedure-related adverse events. Shortterm physiological changes were minimal for both interventions, and WOB and neurological status remained stable, suggesting that the procedures were tolerated and did not cause significant patient discomfort. These findings support the operational feasibility of the NIOD in the ICU and inform the design of a subsequent definitive trial not restricted to COVID-19. Prior studies of airway clearance, including oscillatory devices, have largely focused on chronic conditions such as cystic fibrosis (CF), with mixed evidence and no consistent superiority of one technique over another [8-10]. Evidence in critically ill adults remains sparse. Our results extend this literature by demonstrating that NIOD can be protocolized and delivered by non-physiotherapist personnel in a critical care setting without apparent safety signals, while short-term physiologic effects appear limited.
This pilot study has limitations. First, as a pilot study, the sample size was small and was not powered to detect differences in clinical outcomes. Therefore, all findings regarding physiological parameters should be interpreted as exploratory. Second, it was conducted at a single center with trained non-physiotherapist personnel (ICU nurses and respiratory therapists), which may limit generalizability to settings without similar staffing or training. Third, the cohort comprised only patients with COVID‑19. However, these patients represent a population with severe acute respiratory failure. Fourth, we evaluated only a single session of each intervention, and potential cumulative or longer-term effects were not assessed. Fifth, while the three-hour washout period was chosen, we cannot exclude the possibility of carry-over effects. Finally, the specific NIOD device used in this study (Frequencer®) is no longer commercially available.
However, the underlying principle of non-invasive external chest-wall oscillation is shared by other available devices. While we cannot assume that the safety and feasibility profile would be identical across different models, our results provide valuable proof-of-concept evidence. Given CPT’s adjunctive role, future trials of currently available oscillatory devices should benefit from focusing on short-term, mechanism-proximal outcomes (e.g., sputum clearance, suctioning requirements, radiographic or lung ultrasound–based atelectasis scores, short-term oxygenation indices, and respiratory mechanics), while collecting distal clinical outcomes as secondary or exploratory measures. If confirmed in definitive trials, adopting oscillatory devices as an alternative to cCPT could enable more frequent airway‑clearance sessions when physiotherapy staffing is limited and may reduce healthcare workers’ exposure in rooms housing contagious patients.
Conclusions
NIOD delivered by non‑physiotherapist staff was feasible and appeared safe in critically ill adults; definitive trials are needed to assess clinical impact.
Acknowledgement
We thank all our colleagues for their valuable assistance during the data collection process. Expressions of gratitude are particularly extended to the nurses and respiratory therapists who utilized NIOD in this study to treat patients, particularly in light of their contributions during the challenging period of the pandemic. Appreciation is also conveyed to Dr. Jacques Lacroix for his guidance in the study’s design. Additionally, gratitude is directed towards all the physical therapists for their active involvement in the study.
Contributor’s Statement
AK, KK, and PJ conceptualized and designed the study and data collection protocols. AK, LC, MC, and TL contributed to collecting data, MM and AK carried out initial analyses, MM and AK drafted the initial manuscript, and all the authors revised the initial manuscript and approved the final manuscript as submitted.
Conflict of Interests
None.
Sponsor & Funding Source
Ministère de l’Économie et de l’Innovation et de l’Énergie, Gouvernement du Québec
Financial Disclosure
Ministère de lÉconomie et de lInnovation et de lÉnergie, Gouvernement du Québec
Clinical Trial Registration
ClinicalTrials.gov ID NCT04361435.
The Institution where the study was performed
Royal Victoria Hospital, Montreal, Canada.
References
- Xiaomei Chen, Jiaojiao Jiang, Renjie Wang, Hongbo Fu, Jing Lu, et al. (2022) Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev 9(9): CD006338.
- Denise Battaglini, Chiara Robba, Salvatore Caiffa, Lorenzo Ball, Iole Brunetti, et al. (2020) Chest physiotherapy: An important adjuvant in critically ill mechanically ventilated patients with COVID-19. Respir Physiol Neurobiol 282: 103529.
- Thomas A Scherer, Jurg Barandun, Elena Martinez, A Wanner, E M Rubin (1998) Effect of High-Frequency Oral Airway and Chest Wall Oscillation and Conventional Chest Physical Therapy on Expectoration in Patients with Stable Cystic Fibrosis. CHEST 113(4): 1019-1027.
- Hsiao-Ping Huang, Kee-Hsin Chen, Chen-Liang Tsai, Wen-Pei Chang, Sherry Yueh-Hsia Chiu, et al. (2022) Effects of High-Frequency Chest Wall Oscillation on Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Chron Obstruct Pulmon Dis 17: 2857-2869.
- Morrison L, Innes S (2017) Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev 5(5): CD006842.
- Long H Nguyen, David A Drew, Mark S Graham, Amit D Joshi, Chuan-Guo Guo, et al. (2020) Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 5(9): e475-e483.
- Shadman Aziz, Yaseen M Arabi, Waleed Alhazzani, Laura Evans, Giuseppe Citerio, et al. (2020) Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med 46(7): 1303-1325.
- Federico Longhini, Andrea Bruni, Eugenio Garofalo Chiara Ronco, Andrea Gusmano, et al. (2020) Chest physiotherapy improves lung aeration in hyper secretive critically ill patients: a pilot randomized physiological study. Crit care 24(1): 479.
- Kevin J O’Sullivan, Valerie Power, Barry Linnane, Deirdre McGrath, Hilda Fogarty, et al. (2021) An initial evaluation of the safety of a disposable oscillating positive expiratory pressure device in patients with chronic obstructive pulmonary disease: a short‑term pilot study. BMC Pulm Med 21(1): 326.
- Main E, Rand S (2023) Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database Syst Rev 5(5): CD002011.
-
Marika Matsumoto MD*, Atsushi Kawaguchi MD PhD, Kosar Khwaja MD MBA, Matthew P Cheng MD, Todd Lee MD MPH and Philippe Jouvet MD MBA PhD. Non-Invasive Oscillating Device Use as Chest Physiotherapy in Critical Care, Comparing Conventional Chest Physiotherapy A Pilot Crossover Randomized Controlled Trial. Annal Biostat & Biomed Appli. 6(5): 2025. ABBA.MS.ID.000649.
Pilot study; crossover-randomized-trial; chest physiotherapy; non-invasive oscillation device; critical care; COVID-19; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






