 Review Article
Review Article
Liquid Crystal-Based Biosensors
Sepideh Norouzi and Monirosadat Sadati*
Department of Chemical Engineering, Swearingen Engineering Center, University of South Carolina, Columbia, United States
Monirosadat Sadati, Department of Chemical Engineering, Swearingen Engineering Center, University of South Carolina, Columbia, United States.
Received Date: August 05, 2021; Published Date: August 31, 2021
Abstract
Prolific research toward the development and modification of biosensing platforms is expanding the horizon of clinical diagnosis, health monitoring and drug diagnosis. Most biosensors performance relies on sophisticated instrumental analysis, which is costly, time-consuming and involves additional complications and risks. Liquid crystal biosensors have been considered as an efficient alternative to conventional sensing methodologies. Due to the orientation-dependent interactions at their interface, they can be designed to detect specific biomolecular entities and prevent the need for sophisticated instrumental analysis. This review summarizes the studies on liquid crystalline materials for biosensing applications from molecular elaboration and design perspectives.
Introduction
The outbreak of newly mutated viruses and infectious diseases has driven extensive efforts to develop novel biosensors or improve current sensing platforms. The main challenges associated with the available technologies include their slow-readout, laborintensive, less sensitive, and less selective performance [1-3]. In addition to recognizing immunodeficiency viruses, biosensors are extensively used in healthcare, drug diagnosis, food processing, and environmental monitoring [4-6]. Two broad categories of biosensors include sophisticated laboratory-based instrumental techniques and easy-to-use, portable, and inexpensive devices [4]. Later which has the large-scale consumer market, plays a crucial role in the biosensing industry. Among available technologies, liquid crystal (LC)-based sensing devices have been demonstrated to be reliable platforms for detecting the chemical and biochemical binding events. LCs are states of matter that represent the combined properties of solid crystals and liquids concurrently [7]. The unique hybrid liquid-order properties make LCs responsive to molecular-scale events at the interface. Small perturbations in the LC molecules alignment at the interface can propagate through the bulk resulting in distinct optical features [8-9].
Sensitivity, fast and easy-reading optical responses make LCs attractive candidates for developing LC-based noninvasive, inexpensive miniaturized sensing devices. In this work, liquid crystalline materials in terms of their orientational principles and mesophases structures will be explained. Then the application of LCs in biosensing will be briefly reviewed.
Liquid Crystalline Mesophases
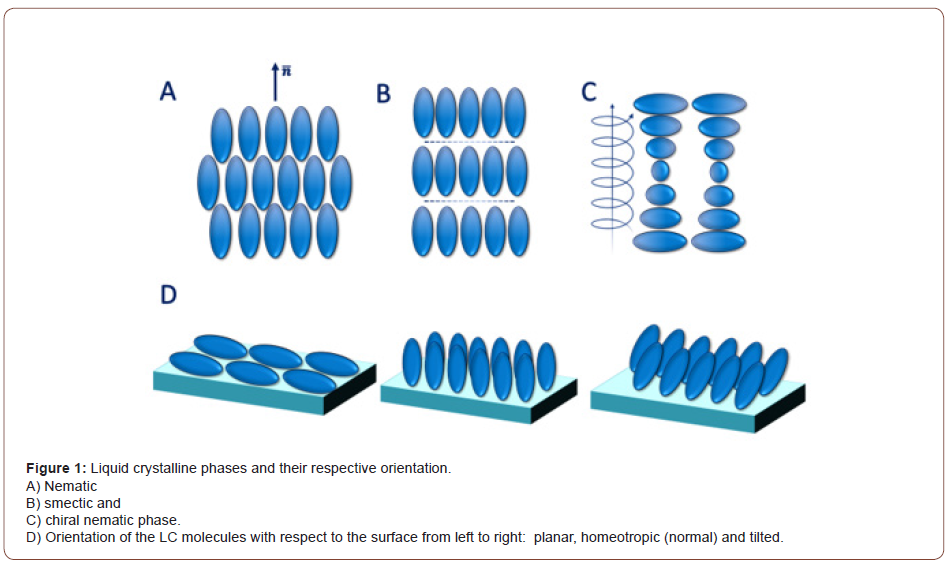
LCs, made of rod shape or disc shape molecules, are categorized into two major classes: thermotropic and lyotropic. While thermotropic liquid crystalline phases appear in a specific temperature range, lyotropic LCs form at a particular concentration. In response to external stimuli, the lyotropic LC molecules with large aspect ratios exhibit slow dynamics, which limits their application in the sensing area [10]. Thermotropic LCs appear between perfectly crystal and isotropic phases. Rod-shape thermotropic LCs can arrange into different mesophases including, nematic, smectic, and chiral nematic (Figure 1A-1C). The nematic phase is known as the least ordered mesophase in LCs in which the molecules are mostly aligned along the director field, n ̅ (Figure 1A) [11]. The smectic phase shows both orientational and positional order in which LC molecules are stratified into layers (Figure 1B). In the chiral nematic phase, on the other hand, LC molecules twist with respect to each other and create a submicrometric helical organization (Figure 1C). The distinction between mesophases in LCs can be made by introducing the director field, n ̅. It indicates the average direction along which the molecules tend to align; the spatial deviation of the molecules to the normal axis determines their orientational order. The orientational order in LCs is expressed as:
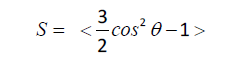
Where Ө is the angle between the director (n ̅) and the long axis of LC molecule. The order parameter in the isotropic and crystal phases are zero and one, respectively. The order parameter of the liquid crystalline mesophases with the positional and orientational order varies between 0.6 to 0.7.
As mentioned earlier, LCs are sensitive to the interfacial phenomena in which their orientation at the interface is controlled by the surface properties. LC molecules at the interface can adopt planar, homeotropic, and tilted configurations (Figure 1D), which are considered as the design principle of LC-based sensors.
Nematic LC
LC as a biosensor was introduced in 1995 by the pioneering work of Abbott et al. [12]. They discovered the responsiveness of the nematic LC interfaces to alkanethiol compounds with different chain lengths. In the other work, Lockwood and Abbott reported the self-assembly of phospholipids at the 4-Cyano-4’- pentylbiphenyl (5CB) LC- aqueous interface, which resulted in the planar to homeotropic orientation transition [13]. The packing of the phospholipids at the interface was then disturbed by protein bindings and enzyme-catalyzed reactions, leading to significant change in the LC molecules’ orientation and their optical features [13]. Since then, the nematic LC films have been employed to detect various types of bioanalytes, including biomolecular assay, protein assay, DNA, and other biological entities [14-16].
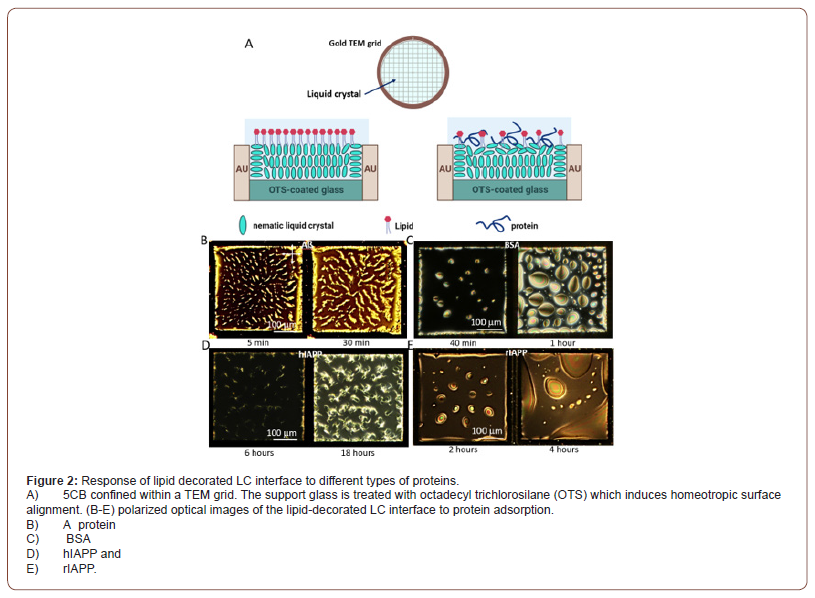
Sadati et al. have shown that the lipid decorated 5CB film confined within TEM grids (Figure 2A) can serve as a sensitive sensor to detect protein aggregation and 𝛽-sheet formation at the oligomer state. This unique feature can be further used for the early-stage diagnosis of neurodegenerative diseases (Figure 2) [17]. They have found different order transitions in 5CB in contact with various types of protein, including A𝛽 amyloid (Figure 2B), bovine serum albumin, BSA (Figure 2C), human islet amyloid polypeptide, hIAPP (Figure 2D), and rat islet amyloid polypeptide, rIAPP (Figure 2E). Their results reveal that proteins and peptides with 𝛽-sheet amyloid structure generate higher anchoring energies and form branch-like structures on the lipid-decorated LC interfaces. In contrast, those with mainly α-helical character exhibit weak anchoring at the interface and phase separate to form circular domains of protein/peptide (Figure 2B) [17].
According to Wang et al., the 5CB interface stabilized by a nonionic surfactant named dodecyl-𝛽-D-glucopyronoside is able to detect different types of proteins including, lysozyme, concanavalin A and bovine serum albumin by enzymatic hydrolysis and saccharide-binding mechanisms [18]. They have also found that the sensitivity of the 5CB interface varies in response to different protein concentrations; for example, 0.01 μg/ml is sufficient to trigger the orientation of the LC molecules at the interface, which is one order of magnitude smaller than other proteins. Verma et al. have investigated the response of polymyxin B (PmB) laden- 5CB interface to different protein structures [19]. Exposing the immobilized PmB on the LC interface to anionic bovine serum albumin triggered the fibril-like textures formation at the 5CBaqueous interface which was not observed in helix rich counterpart [19]. LCs have also been used for immunoassay and gene detection. Shen et al. have shown that the nematic LCs undergo the orientational transitions depending on the DNA type [20]. While a single-strand DNA does not interfere with the homeotropic alignment of 5CB, the hybridized DNA induces the planar orientation. This finding confirms a promise for high precision, label-free, and cost-effective DNA detection [20].
LCs in the form of microdroplets suspended in an aqueous phase hold great advantages over LC films for sensing applications. The benefits of LC microdroplets include a higher surface to bulk ratio, free director field of LC molecules at the interface, and random motion of LC microdroplets toward targeted bioanalytes, resulting in faster and lower threshold response. In this regard, Lin et al. have found that picograms of endotoxin in a milliliter of water can trigger the ordering transition of liquid crystal microdroplets [21]. Sivakumar et al. fabricated the mono-disperse nematic LC emulsion to detect and distinguish the bacteria and viruses based on their cell-wall structures [22]. They observed the transition from bipolar to planar orientation in the nematic LC droplets upon contact with E. Coli bacteria (Gram ve-) and A/NWS/Tokyo/67 enveloped viruses, while no transition in orientational order was observed with Gram ve+ bacteria and non-enveloped viruses [22].
Chiral Nematic LC
In chiral liquid crystals (CLC), the macroscopic helical orientations can be easily triggered by the biological analytes, leading to a faster response within the orders of milliseconds. This feature has been employed in optoelectronic devices and technologies. Moreover, chirality gives rise to various configurational orders that appeal to sensing applications and can be used to distinguish various biochemical analytes. Despite their extensive applications in the optoelectronic area, the potential of CLCs in biosensing has not been thoroughly explored. Thus, there is a need to study the interfacial interaction of this class of LCs with biological analytes.
Hsiao et al. [22] reported the response of homeotropically aligned CLC films to bovine serum albumin. Their results exhibit considerably higher sensitivity of the CLC-aqueous interface than achiral systems. A lower concentration of protein (1 fg/ml) can trigger ordering transition at the interface leading to distinct color change. The pallets of colors that appeared at different amounts of the protein indicate that a CLC-aqueous interface can be used as a quantitative measure of concentration. Kyung-Gyu Noh and Soo-Young Park [23,24] developed the urea sensor array based on an interpenetrating polymer network (IPN) of CLC and enzyme immobilized polyacrylic acid (PAA). They have observed a color change in CLC-based IPN upon expansion and shrinkage of the PAA network. Lee et al. [25] employed the microfluidic technique to fabricate CLC droplets coated with poly (acrylic acid)-b-poly (4-cyanobiphenyl-4′-oxyundecyl acrylate). They immobilized droplets by glucose oxidase and cholesterol oxidase for detection of glucose and cholesterol, respectively. The mechanism behind this sensing approach is protonation and deprotonation of a carboxylic acid group which induces the planar and homeotropic orientations, respectively. In a different study, Concellon et al. [26] found that the addition of IgG antibodies to a LC emulsion stabilized by boronic acid surfactant can change the chiral pitch length leading to different color reflections.
Conclusion
Overall, research findings confirm that LC interfaces are reliable reporters of their interfacial phenomena, enabling fast detection of various bio-analytes. The LC-based sensors are sensitive and rapid and can operate at room temperature without complex instrumentation. They provide a platform to design portable, flexible, and simple devices to use. Hence LCs can be easily incorporated into wireless and wearable optoelectronic devices.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Mehrotra P (2016) Biosensors and their applications–A review. J Oral Biol Craniofac Res 6(2): 153-159.
- Chen C, Wang J (2020) Optical biosensors: An exhaustive and comprehensive review. Analyst 145(5): 1605-1628.
- Chambers JP, Arulanandam BP, Matta LL, Weis A, Valdes JJ (2008) Biosensor recognition elements. Curr Issues Mol Biol 10(1-2): 1-12.
- Turner AP (2013) Biosensors: sense and sensibility. Chem Soc Rev 42(8): 3184-3196.
- Mello LD, Kubota LT (2002) Review of the use of biosensors as analytical tools in the food and drink industries. Food chemistry 77(2): 237-256.
- Goode JA, Rushworth JV H, Millner PA (2015) Biosensor regeneration: a review of common techniques and outcomes. Langmuir 31(23): 6267-6276.
- Andrienko D (2018) Introduction to liquid crystals. Journal of Molecular Liquids 267: 520-541.
- Wang Z, Xu T, Noel A, Chen YC, Liu T (2021) Applications of liquid crystals in biosensing. Soft Matter 17(18): 4675-4702.
- Esteves C, Ramou E, Porteira ARP, Moura Barbosa AJ, Roque ACA (2020) Seeing the unseen: the role of liquid crystals in gas‐sensing technologies. Advanced optical materials 8(11): 1902117.
- Giese M, Blusch LK, Khan MK, MacLachlan MJ (2015) Functional materials from cellulose‐derived liquid‐crystal templates. Angewandte Chemie International Edition 54(10): 2888-2910.
- Murphy A (2011) Blue Phases in Liquid Crystals.
- Drawhorn RA, Abbott NL (1995) Anchoring of nematic liquid crystals on self-assembled monolayers formed from alkanethiols on semitransparent films of gold. the Journal of Physical Chemistry 99(45): 16511-16515.
- Lockwood NA, Abbott NL (2005) Self-assembly of surfactants and phospholipids at interfaces between aqueous phases and thermotropic liquid crystals. Current Opinion in Colloid & Interface Science 10(3-4): 111-120.
- Khan M, Khan AR, Shin JH, Park SY (2016) A liquid-crystal-based DNA biosensor for pathogen detection. Sci Rep 6:22676.
- Wu PC, Karn A, Lee MJ, Lee W, Chen CY (2018) Dye-liquid-crystal-based biosensing for quantitative protein assay. Dyes and Pigments 150: 73-78.
- Popov P, Mann EK, Jákli A (2017) Thermotropic liquid crystal films for biosensors and beyond. Journal of Materials Chemistry B 5(26): 5061-5078.
- Sadati M, Apik AI, Armas Perez JC, Martinez Gonzalez J, Hernandez Ortiz JP, et al. (2015) Liquid crystal enabled early-stage detection of beta amyloid formation on lipid monolayers. Advanced Functional Materials 25(38): 6050-6060.
- Wang Y, Hu Q, Tian T, Yan’an Gao, Yu L (2016) A nonionic surfactant-decorated liquid crystal sensor for sensitive and selective detection of proteins. Analytica chimica acta 937: 119-126.
- Verma I, Rajeev N, Mohiuddin G, Pal SK (2019) Ordering transitions in liquid crystals triggered by bioactive cyclic amphiphiles: Potential application in label-free detection of amyloidogenic peptides. The Journal of Physical Chemistry C 123(11): 6526-6536.
- Shen J, He F, Chen L, Ding L, Liu H, et al. (2017) Liquid crystal-based detection of DNA hybridization using surface immobilized single-stranded DNA. Microchimica Acta 184(9): 3137-3144.
- Lin IH, Miller DS, Bertics PJ, Murphy CJ, de Pablo JJ, et al. (2011) Endotoxin-induced structural transformations in liquid crystalline droplets. Science 332: 1297-1300.
- Sivakumar S, Wark KL, Gupta JK, Abbott N, Caruso F (2009) Liquid crystal emulsions as the basis of biological sensors for the optical detection of bacteria and viruses. Advanced Functional Materials 19(14): 2260-2265.
- Hsiao YC, Sung YC, Lee MJ, Lee W (2015) Highly sensitive color-indicating and quantitative biosensor based on cholesteric liquid crystal. Biomed Opt Express 6(12): 5033-5038.
- Noh KG, Park SY (2018) Biosensor Array of Interpenetrating Polymer Network with Photonic Film Templated from Reactive Cholesteric Liquid Crystal and Enzyme‐Immobilized Hydrogel Polymer. Advanced Functional Materials 28(22): 1707562.
- Lee HG, Munir S, Park SY (2016) Cholesteric liquid crystal droplets for biosensors. ACS Appl Mater Interfaces 8(39): 26407-26417.
- Concellón A, Fong D, Swager TM (2021) Complex Liquid Crystal Emulsions for Biosensing. J Am Chem Soc 143(24):9177-9182.
-
Sepideh Norouzi and Monirosadat Sadati. Annal Biostat & Biomed Appli. 4(3): 2021. ABBA.MS.ID.000590. DOI: 10.33552/ABBA.2021.04.000590
Clinical diagnosis, Biosensors, Drug diagnosis, Gene detection, Instrumental analysis, Immunodeficiency viruses, Liquid crystalline materials
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






