 Research Article
Research Article
Differences in the Distribution of Aβ in the Brain between U.S. Veterans and Adults aged 62+ and suffering from Alzheimer’s Disease
Stanislav Kolpakov Nikitin*, Arseniy Yashkin and Igor Akushevich
Social Science Research Institute, Duke University, Durham
Stanislav Kolpakov Nikitin, Social Science Research Institute, Duke University, Durham
Received Date: June 16, 2024; Published Date: June 26, 2024
Abstract
Introduction: An elevated concentration of amyloids in the cerebrum results in elevated risks for cerebral hemorrhage and early AD onset following early depression/dementia onset. In this study, we compare patterns of amyloid depositions across eight regions of interest of the human brain between U.S. Veterans and non-Veterans adults aged 62+.
Data: were taken from the ADNI and DoD-ADNI studies. The pool of participants included data about age, race, apolipoprotein ε4 allele (APOE) status, modified Hachinski Ischemic Score, education level, and the Geriatric Depression Score, which were used to build a propensity score. Predictors and outcomes were Aβ concentrations, resulting from the PET image analysis taken in key brain regions of interest, and two categorical variables describing the 0.79 and 1.11 cutoffs were used as outcomes, in addition, the Veteran and AD status were used as predictors.
Methods: To balance subsamples, we applied a pseudo-randomization algorithm, eliminating the observed sources of heterogeneity. We used a generalized linear model for continuous variables and the logistic regression model for binary variables.
Results and Conclusion: illustrate that the pattern of the Aβ distribution in Veteran’s brains differs from the Aβ distribution pattern in the brain of those who live with AD. The amyloid depositions following Veteran status are concentrated in the cerebellum, particularly in cerebellar gray matter. In contrast, the AD pattern shows more Aβ depositions in the frontal lobe, cingulate cortex, parietal, and temporal lobes, along with higher whole-cerebrum concentration of amyloids. Since Florbetapir PET cannot distinguish between senile plaques and depositions in blood vessels, the elevated concentration of amyloids in a cerebellum for participants with the Veteran status may suppose elevated risks for cerebral hemorrhage and early AD onset following early depression/dementia onset.
Keywords:Alzheimer’s disease neuroimaging initiative; traumatic brain injury; post-traumatic stress disorder
Introduction
The reduction of the social impact of Alzheimer’s disease (AD) [1- 2] supposes substantial cost savings and improvement of the life quality of the older segment of population. There is currently no cure for AD, and a newly diagnosed individual age 65+ can expect to live for as long as 10 years or more after diagnosis [3-5]. The additional burden associated with AD can start accumulating up to two years before clinical diagnosis [6]. The related socio-economic load increases with severity of the disease, requiring use of highintensity nursing facilities and skilled care during the final two to four years of life [7]. Excluding intrinsic genetic risks, age is the next critical risk factor for AD, given the most of individuals diagnosed with AD exceed the age of 65. In the U.S., this population of older adults is increasing in both size and life expectancy. At the same time, mortality stemming from many age-related conditions (as well as other prominent sources of mortality like cancer) has been mitigated and/or delayed by improving methods of disease management and treatment. This has led to an ascent in the number of older adults with AD diagnostic, and the need to plan for longer survivorship in such individuals, in the presence of multiple potentially risk-dependent chronic conditions [8-12].
Veterans of the U.S. Armed Services represent an important subgroup of the general population of older adults age 65+. Approximately, 9 million strong in 2021, this population reflects a unique demographic (96.37% Male; 3.63% Female) and health characteristics [13] both positive (e.g. the healthy soldier effect) [14] and negative (e.g. service-related injuries, exposure to chemicals, trauma, etc.) [15-17] in nature. There is a scarcity of research directly addressing differences in AD and/or dementia prevalence between comparable groups of veterans and civilians. However, a recent comparative study has shown that once differences in health, demographics, and socioeconomic status between veterans and non-veterans are accounted for, these subgroups demonstrate comparable AD risk [18-22]. In this study, we compare patterns of amyloid depositions in the human brain, across eight regions of interest (ROI), between U.S. Veterans and non-veteran adults aged 62+ and discuss how the differences in the physiological changes may be indicative of early AD onset or other issues related to the deterioration of a human brain.
Methods
Data
This study was based on data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) is a longitudinal study aimed at creating biochemical, genetic, imaging, and clinical biomarkers database for the investigation of the possibility of early identification and monitoring the progression of AD. ADNI data were collected in three stages (ADNI-1, ADNI-GO, ADNI-2). Although, at the time of finalization this study, data from the fourth stage (ADNI-3) were still not fully available. An ADNI extension study sponsored by the Department of Defense (ADNI-DoD) identifies the Veteran population and provides an extended range of measures specific to this subgroup such as traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), and adverse health-related factors derived from military service. The DoD-ADNI contains medical and demographic data on Veterans of the Vietnam War identified from the Veteran Affairs Compensation and Pension records and have magnetic resonance imaging (MRI), amyloid PET using Florbetapir-18 (F18), cognitive testing through a telephone interview, cerebral spinal fluid biomarkers of tau, phosphorylatedtau, Aβ, and blood sampling for analysis of genetic factors.
The PET protocol for ADNI-DOD was the same as for all ADNI stages, thus our analysis does not require harmonization. A cortical summary region of interest (ROI) was composed of frontal, anterior and posterior cingulate, lateral parietal, and lateral temporal ROIs. The MRI scans for each ROI were processed using Free Surfer v7.1.1 [23]. The cerebellar grey matter, whole cerebellum, brainstem and the pons, eroded subcortical white matter, a composite reference region made up of whole cerebellum, brainstem and the pons, and eroded subcortical white matter regions were defined as the candidate references. Each Florbetaben scan was co-registered with the corresponding MRI to estimate the mean amyloid PET uptake within the cortical and reference regions [24]. For participants with more than one PET, we selected the image taken as close as possible to the date when all the analyses were finished. This PET processing and analysis pipeline is consistent with the UC Berkeley AV45 pipeline. The acquisition of neuropathology data for the ADNI database was made according to the guidelines for the neuropathologic assessment of Alzheimer’s disease coined by National Institute on Aging and Alzheimer’s Association [25].
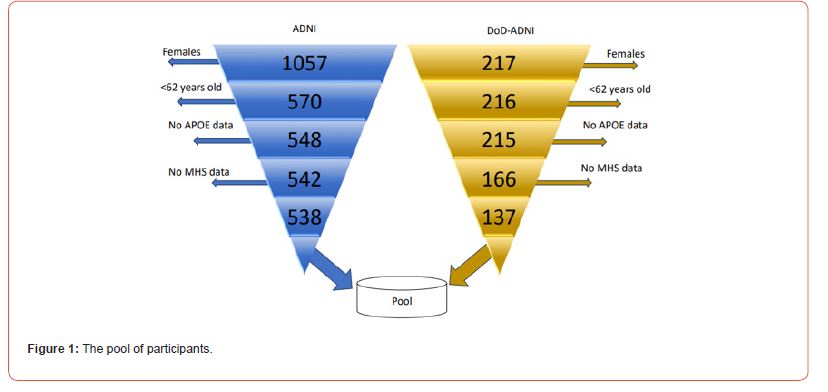
Pool and Subsamples
The initial database sizes were 4,207 individuals for ADNI-DOD and 6,759 for ADNI. After restrictions (Figure 1), these numbers were reduced to a sample of 675 male adults aged 62+, including 137 Veterans, and 538 non-un veterans. The AD patient was considered if the diagnostic was confirmed before the PET image was taken. The poll covered a total of 123 AD cases, 24 of them correspond to the U.S. Veterans and 99 to not veterans. Finally, the pool was split into two subsamples: The subsample (S1) encompassed 538 individuals with and without AD. This sample was used to outline the amyloid pattern in the brains of an average patient with AD. The subsample (S2) contained 113 U.S. Veterans and 439 not veterans. The subsample S2 was used to sketch the amyloid pattern in the brains of an average U.S. Veteran in the absence of an AD diagnostic.
Outcomes
Outcomes included eight regions of interest (ROI) of the brain: (X1) cerebellar gray matter; (X2) whole cerebellum; (X3) eroded subcortical white matter; (X5) frontal lobe; (X6) cingulate cortex; (X7) parietal lobe; (X8) temporal lobe; (X11) brainstem; and four auxiliary summary measures: (X4) unweighted composite average of the whole cerebellum, brainstem and the pons, and eroded subcortical white matter regions, (X9) composite average of the whole cerebellum, brainstem regions brainstem and the pons and eroded subcortical white matter regions; (X10) summary over whole cerebrum cortical composite region, normalized by the Free Surfer-defined composite reference region, and (X12) composite reference summary. We also considered two categorical variables (X13 and X14) with a value of zero if the summary over the whole cerebrum (X10) is below a cutoff threshold of 0.79 or 1.11, respectively, and is one, otherwise [26,27].
Variables
We used the following variables: age at the time of the PET scan, race, sex, education level, the geriatric depression score (GDS), APOE-ε4 allele status, Veteran status, and the Modified Hachinski Score which indicates the likelihood of dementia due to ischemic causes.
Statistical Analyses
Before running a pattern detection, we reduced the effects of observed confounding by applying a pseudo-randomization algorithm [28-30] (see results in Table 1). The algorithm used the AD-related variables: age at the time of the PET scan, race, biological sex, education level, GDS, APOE-ε4 allele status, the U.S. Veteran status, and the Modified Hachinski Score which indicates the likelihood of dementia due to ischemic causes. An inverse probability of treatment weighting (IPTW) was calculated for each observation as the reciprocal of the probability of the event (e.g. AD diagnostic for S1 or Veteran status for S2). For continuous variables (X1-X12), we used the generalized linear regression model. Meanwhile, for categorical variables X13 and X14, we applied the logistic regression model. Finally, the patterns of amyloid distribution in the average U.S. Veteran brain and the average brain affected by AD were established and compared. The Duke University, Institutional Review Board, approved the protocol used in this study. All analyses were conducted using R and SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
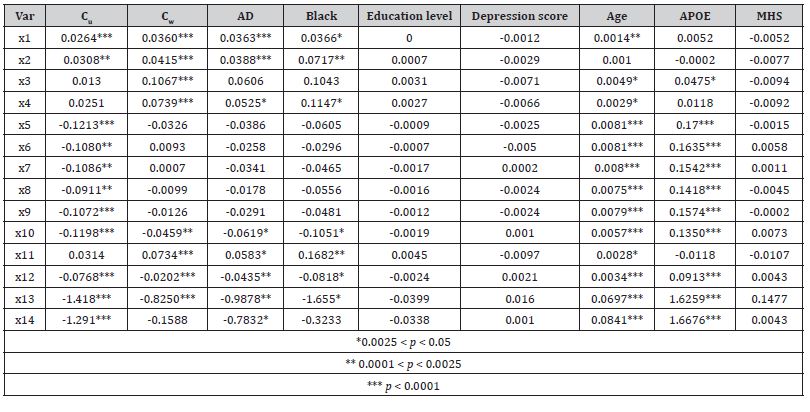
Table 1 illustrates the subsamples summary and the quality of the pseudo-randomization. Before pseudo randomization, in the subsample S1, the AD-affected and “healthy” groups differed significantly by GDS, age at the time of PET scan, and APOE-ε4 allele status. Similarly, in the S2, the Veteran and non-veteran groups differed significantly by race, education levels, GDS, age, and APOE status. After pseudo-randomization, all observed statistically significant differences were mitigated. The amyloid patterns for the fourteen ROI measures used in this study are shown in Table 2 (for S1) and Table 3 (for S2). In both tables, the column labeled Cu shows betas for the unweighted univariate analysis (i.e. all other possible predictors are at the population average), while the column labeled with Cw illustrates the results of the IPW-adjusted model. The pattern of the Aβ distribution in Veteran’s brains was found to be different from the classic AD pattern.
Statistically significant positive differences in concentrations of amyloid deposits between Veterans and not Veterans (Table 2) were observed for cerebellar gray matter (X1), whole cerebellum (X2), and eroded subcortical white matter (X3). Moreover, while the amyloid depositions following Veteran status were concentrated mostly in the cerebellum eroded subcortical gray matter and brainstem, the density of depositions in the whole cerebrum (X10) was less than for non-veterans. In contrast, the AD pattern (Table 3) supposes that the most important amyloid depositions occur in the frontal lobe (X5), cingulate cortex (X6), parietal lobe (X7), temporal lobe (X8), also indicating a significant overall increase in brain located amyloids (X13 and X14). The concentration of Aβ in the brainstem (X11) was significantly lower in AD patients than in their healthy counterparts (Table 2). Similarly, for both patterns, race, age, and APOE-ε4 allele status were found to exercise significant influence over Aβ depositions. As expected, Black participants have shown more deposits, which supposes faster deterioration of normal functions.
Table 1: The summary statistics and significance and pseudo-randomization quality testing for variables involved in subsamples S1 and S2.
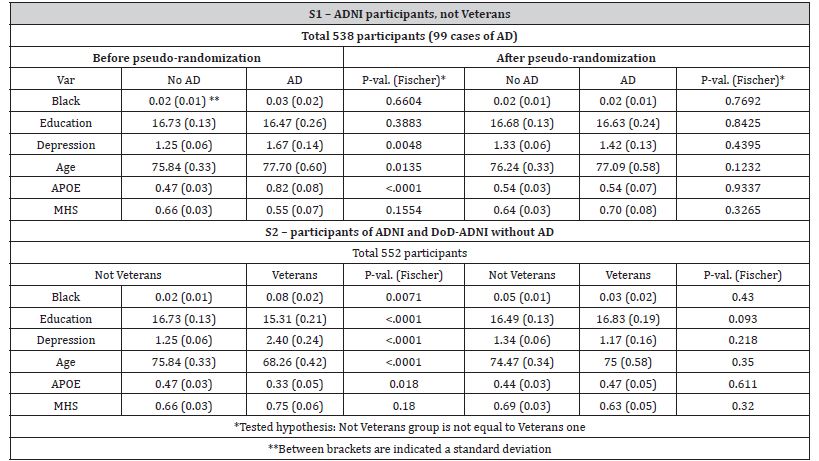
Table 2: The pattern of Aβ deposits in the average brain of not Veteran with AD versus the brain without AD.
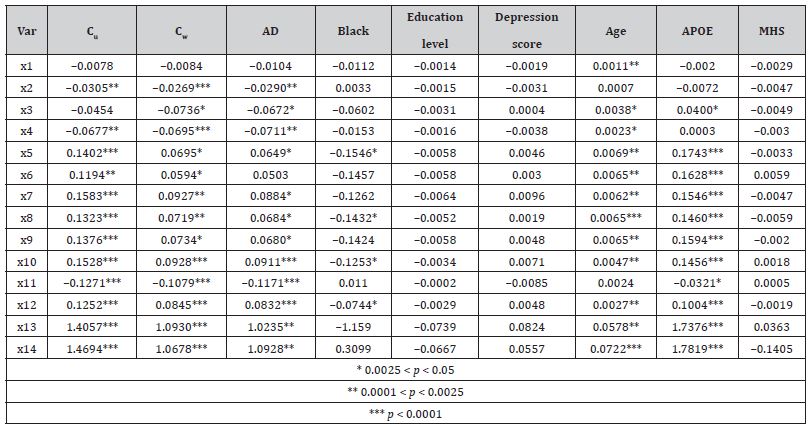
Table 3: The pattern of Aβ deposits in the average brain of a veteran versus not-veteran without AD.
Discussion
It is helpful to have a conceptual model of the long-term impact of military service on aging to explore various outcomes and factors that may come into play as Veterans grow older. Situating analysis within this framework, we can gain a deeper understanding of the unique challenges faced by Veterans and how we can best support them to keep moving forward 13. Recent data from the U.S. Census Bureau in November 2021 reveals that there are 8,915,189 Veterans aged 65 and over in the United States, comprising 8,591,964 men and 323,225 women. This vital population subgroup warrants close analysis of their health and aging-related characteristics, particularly since an additional 3,176,620 Veterans are expected to join this cohort by 2029. Military service is a significant decision with far-reaching career development and aging implications. It is associated with various health-related factors, both positive (such as discipline and the healthy soldier effect 14) and negative (including service-related injuries 15, exposure to chemicals 16, and diverse types of contusions 17), many of which are often not measured in population studies.
Forty-one percent of U.S. Veterans may potentially require mental health care, according to the Department of Veterans Affairs. According to the survey, 28% of OEF/OIF/OND Veterans admitted to receiving at least one mental health diagnosis within the previous two years. Nearly seven out of ten people who had a positive result on the mental health screeners reported that they received a mental health diagnosis [31]. According to the results of the individual mental health screeners, 23% of the U.S. OEF/OIF/ OND Veterans tested positive for PTSD, 16% for major depression, and 13% tested positive on the Kessler six-item measure of general psychological distress, in the committee’s survey. Three percent tested positive for drug abuse and five percent tested positive for alcohol dependence. Nonetheless, in the 2013 survey, 21% of veterans tested positive for major depressive disorder (MD), 27% for alcohol abuse, and 20% for PTSD. The differences in the cut-off points that each study employed to score the Alcohol Use Disorder Identification Test scale, which evaluates drinking problems, are probably the cause of the disparity in the percentage of participants who scored positively for problems with alcohol.
Recent studies draw attention to the role of alternative neurodegenerative processes, not associated with AD specifically [32-34], but sharing similar manifestations, and potentially leading to similar levels of cognitive decline. Although the factors controlling the onset and progression of extracellular amyloidosis remain mainly unknown [35], specific risks associated with military duty such as stress response [36], exposition to pollution [37], and either constant micro traumatic brain injury (TBI) which military personnel may have as a result of continuous exposition to shock waves or TBI due to head wounds [38], as well as the age-related neurodegeneration processes, are associated with the deposition of amyloid-β plaques [39-42]. It is still unknown if the amyloidosis pattern resulting from the cases associated with harmful war environments is identical to those found in AD patients [43]. After statistically supported investigation, we found that the Aβ distribution in the average brain of the AD-free U.S. Veteran differs from the pattern observed in a comparable population of individuals who were not exposed to a war environment and were diagnosed with AD.
This finding is consistent with the study that found no statistically significant differences in incident TBI or incident clinical AD between “Veteran” and “non-veteran” populations after proper accounting and statistical correction of the differences in socioeconomic and health-related risk factors. Florbetapir is a radiopharmaceutical tracer employed for PET scanning that contains radionuclide fluorine-18 and is approved for use in the U.S. It binds to Aβ and has a half-life of 109.75 minutes, which allows the compound to accumulate in the brain of participants, mainly in the regions associated with Aβ deposits. The 18F images consisted of four frames with 5-minute exposition, taken 50-70 min postinjection of the compound; the frames were realigned, averaged, resliced to a voxel size of 1.5 mm3, and smoothed to resolution of 8 mm3 in full width at half maximum. The dynamic 3D PET scans were performed by injecting 370 MBq (10 mCi) of 18F. All the variables of interest, except categorical ones, were normalized as a standardized uptake value ratio (SUVR). SUVR quantifies the amount of 18F uptake using an unspecific binding of each patient as the reference.
As a reference region (RR) for ROI was taken a brain region recognized by previous neuropathology studies as being mainly pathology-free and having biological properties resembling the ROI. To obtain an SUVR value the 18F uptake in each ROI was divided by the uptake over the matching RR. The amyloid β-protein is the principal constituent of vascular amyloid depositions and of an amyloid core of senile plaques (SP) during AD. The pathological changes with AD are typically associated with changes in the limbic system, although existing studies show that the pathologic changes can also implicate parts of the brain dedicated to the control of movement, especially in patients with early-onset dementia 44. The morphology of SPs depends on the presence of neurofibrillary tangles (NFT) located in the same area of the brain [44]. NFTs are insoluble twisted fibers, primarily of a protein called tau, found inside the brain’s cells [45]. When both NFTs and SPs are present in the same area of the brain, a high fraction of the SPs contains degenerated neurites. Though, in the absence of NFTs, the most common are diffuse plaques [46]. A neurotic SP is a complex structure made of both neuronal and non-neuronal elements. The neuronal part of the SP consists of dystrophic, deteriorating, and regenerating neurites.
The other components of SPs are amyloid deposits, microglia, macrophages, and reactive astrocytes. Although the SPs are particularly numerous in AD, these lesions are also associated with Creutzfeldt-Jacob disease, Gerstmann–Sträussler–Scheinker syndrome, and scrapie in sheep and goats which is caused by infectious agents. The SPs were found in normal-aged human [47- 49] and animal [50] brains. However, the composition of the amyloid fibers in SPs observed for aforementioned diseases is different from the protein composition of SPs in AD and normal aging in humans [51]. However, the concentration of amyloids differs insignificantly in eroded subcortical white matter (compare variable X3 in Tables 2&3). The whole pattern of the Aβ distribution across the Veteran’s brain differs significantly from that found in individuals with AD. The concentration of amyloid depositions following Veteran status is lower in the frontal lobe; cingulate cortex; parietal lobe; temporal lobe, a composite average of the whole cerebellum, brainstem regions brainstem and the pons, eroded subcortical white matter regions, and summary over whole cerebrum cortical composite ROI, and composite reference summary (compare X5- X10, X12-X14) in Tables 2&3), which is typical for AD and with the time leads to unevenness in the shapes of cortical and subcortical structures which, in turn, correlate with severity of cognitive disorder and may predict the onset of Alzheimer disease [52] and it is significantly higher in the brainstem, cerebellar gray matter and in the cerebellum in general (compare X11, X1, X2, and X4 in Tables 2&3).
Since Florbetapir PET cannot distinguish between senile plaques and depositions in blood vessels, it is impossible to draw a more precise picture of the underlying processes, although amyloid deposition in this area and resulting deterioration may lead to a decline in motion functions for instance slowness of movements, rigidity, resting tremor, gait disturbance and postural instability [53], sleep disorder, and depression [54], along with elevated risk for cerebellar hemorrhage. In contrast, the patterns of Aβ distribution associated with AD, suppose elevated concentration of amyloid depositions in the cortex, parietal, and temporal lobes, along with higher whole-cerebrum concentration of amyloids, leads to memory loss and intrinsic dementia. Moreover, the deterioration of the cingulate cortex due to amyloid deposits (variable X6, Table 2) explains assigning wrong emotions to certain stimuli or events, connecting facial expressions to incorrect emotions, and connecting to their vocalizations, which are typical AD symptoms. The analyses show that the “AD” and “Veteran” patterns do not overlap. Although the pathological changes due to AD are usually associated with changes in the limbic system, existing studies show that the disease can also implicate parts of the brain dedicated to the control of movement, especially in patients with early-onset dementia. Also, the early AD onset in the U.S. Veteran society the observed pattern may be a part of a pathway following early depression derived from irregular sleep and coordination troubles.
Author Contribution
Conceptualization, S.K., and I.A.; writing, and preparation of the manuscript, S.K.; review, editing, updates, A.Y. and I.A.; data preparation, statistical analysis, S.K and I.A. All authors have read and agreed to the final version of the manuscript.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (NIA/NIH) under award numbers R01AG066133, R01AG057801, and the U.S. Department of Defense award W81XWH-20-1-0253.
Conflicts of Interests
The authors declare that there is no conflict of interest.
References
- Alzheimer’s A. 2015 Alzheimer's Disease Facts and Figures (2015) Alzheimer's & Dementia: The Journal Of The Alzheimer's Association 11(3): 332-384.
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM (2013) Monetary costs of dementia in the United States. N Engl J Med 368(14): 1326-1334.
- Brookmeyer R, Corrada MM, Curriero FC, Kawas C (2002) Survival Following a Diagnosis of Alzheimer Disease. Arch Neuro 59(11): 1764-1767.
- Cummings JL, Cole G (2002) Alzheimer Disease. JAMA 287(18): 2335-2338.
- Zhu CW, Sano M (2006) Economic considerations in the management of Alzheimer’s disease. Clin Interv Aging 1(2): 143-154.
- Deb A, Thornton JD, Sambamoorthi U, Innes K (2017) Direct and Indirect Cost of Managing Alzheimer’s Disease and Related Dementias in the United States. Expert Rev Of Pharmacoecon Outcomes Res 17(2): 189-202.
- Stallard E, Kinosian B, Stern Y (2017) Personalized predictive modeling for patients with Alzheimer’s disease using an extension of Sullivan’s life table model. Alzheimers Res Ther 9(1): 1-15.
- Anderson G, Horvath J, Knickman J, Colby D, Schear S, et al. (2013) Chronic Conditions: Making The Case For Ongoing Care 2010. Princeton, NJ, Robert Wood Johnson Foundation's Partnership for Solutions Ref Type: Report.
- Mercer SW, Smith SM, Wyke S, O'Dowd T, Watt GC (2009) Multimorbidity in primary care: developing the research agenda. Fam Pract 26(2): 79-80.
- Wolff JL, Starfield B, Anderson G (2002) Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 162(20): 2269-2276.
- DuGoff EH, Canudas-Romo V, Buttorff C, Leff B, Anderson GF (2014) Multiple chronic conditions and life expectancy: a life table analysis. Med Care 52(8): 688-694.
- Szanton SL, Han HR, Campbell J, Reynolds N, Dennison-Himmelfarb CR, et al. (2020) Shifting paradigms to build resilience among patients and families experiencing multiple chronic conditions. J Clin Nurs 29(19-20): 3591-3594.
- Spiro A, Settersten RA, Aldwin CM (2016) Long-term Outcomes of Military Service in Aging and the Life Course: A Positive Re-envisioning. Gerontologist 56(1): 5-13.
- Niazi FA, Riggs JE (2020) Association of ALS and Military Service: Reflection of Survival Bias due to the “Healthy Soldier Effect”? Mili Med 185(1-2): e5-e7.
- Cummins TL, Dore V, Feizpour A, Krishnadas N, Bourgeat P, et al. (2022) Tau, β-amyloid, and Glucose Metabolism Following Service-Related Traumatic Brain Injury in Vietnam War Veterans: The AIBL-VETS Study. J Neurotrauma 40(11-12): 1086-1097.
- Goldman SM, Weaver FM, Stroupe KT, Cao L, Gonzalez B, et al. (2023) Risk of Parkinson Disease Among Service Members at Marine Corps Base Camp Lejeune. JAMA Neurol 80(7): 673-681.
- Riascos D, Buriticá E, Jiménez E, Castro O, Guzman F, et al. (2013) Neurodegenerative Diversity in human cortical contusion: Histological analysis of tissue derived from decompressive craniectomy. Brain Res 1537: 86-99.
- Peterson K, Veazie S, Bourne D, Anderson J (2020) Association between traumatic brain injury and dementia in veterans: a rapid systematic review. J Head Trauma Rehabil 35(3): 198-208.
- Hudomiet P, Hurd MD, Rohwedder S (2018) Dementia prevalence in the United States in 2000 and 2012: estimates based on a nationally representative study. J Gerontol B Psychol Sci Soc Sci 73(suppl_1): S10-S19.
- Langa KM, Larson EB, Crimmins EM, Faul JD, Levin DA, et al. (2017) A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med 177(1): 51-58.
- Williamson V, Stevelink SA, Greenberg K, Greenberg N (2018) Prevalence of mental health disorders in elderly US military veterans: a meta-analysis and systematic review. Am J Geriatr Psychiatry 26(5): 534-545.
- Yashkin AP, Gorbunova GA, Tupler L, Yashin AI, Doraiswamy M, et al. (2023) Differences in Risk of Alzheimer's Disease Following Later-Life Traumatic Brain Injury in Veteran and Civilian Populations. J Head Trauma Rehabil 38(6): E384-E393.
- Dale AM, Fischl B, Sereno MI (1999) Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage 9(2): 179-194.
- Fischl B, Dale AM (2000) Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc Natl Acad Sci U S A 97(20): 11050-11055.
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123(1): 1-11.
- Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, et al. (2013) Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med 54(1): 70-77.
- Landau SM, Fero A, Baker SL, Koeppe R, Mintun M, et al. (2015) Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med 56(4): 567-574.
- Austin PC (2010) The Performance Of Different Propensity‐Score Methods for Estimating Differences In Proportions (Risk Differences Or Absolute Risk Reductions) in Observational Studies. Stat Med 29(20): 2137-2148.
- Akushevich I, Yashkin AP, Greenup RA, Hwang ES (2020) A Medicare-Based Comparative Mortality Analysis Of Active Surveillance In Older Women With Dcis. NPJ Breast Cancer 6(1): 1-8.
- Akushevich I, Kravchenko J, Arbeev KG, Ukraintseva SV, Land KC, et al. (2016) Health Effects and Medicare Trajectories: Population-Based Analysis of Morbidity and Mortality Patterns. Biodemography of Aging 40: 47-93.
- DoVAMHS (2018) Evaluation of the Department of Veterans Affairs mental health services.
- Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, et al. Association of Traumatic Brain Injury with Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol 73(9): 1062-1069.
- Weiner MW, Crane PK, Montine TJ, Bennett DA, Veitch DP (2017) Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology 89(18): 1923-1925.
- Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, et al. (2017) Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer's disease in Vietnam veterans using the Alzheimer's Disease Neuroimaging Initiative: preliminary report. Alzheimer's Dement 3(2): 177-188.
- Sekijima Y, Wiseman RL, Matteson J, Hammarstrom P, Miller SR, et al. The biological and chemical basis for tissue-selective amyloid disease. Cell 121(1): 73-85.
- Galkin AP, Sysoev EI (2021) Stress response is the main trigger of sporadic amyloidoses. Int J Mol Sci 22(8): 4092.
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, et al. (2008) Long-term Air pollution Exposure is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid β-42 and α-Synuclein in Children and Young Adults. Toxicol Pathol 36(2): 289-310.
- Johnson VE, Stewart W, Smith DH (2010) Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease? Nat Rev Neurosci 11(5): 361-370.
- Lye TC, Shores EA (2000) Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychology Review 10(2): 115-129.
- Blennow K, Hardy J, Zetterberg H (2012) The Neuropathology and Neurobiology of Traumatic Brain Injury. Neuron 76(5): 886–899.
- Smith DH, Johnson VE, Stewart W (2013) Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9(4): 211-221.
- DeKosky ST, Blennow K, Ikonomovic MD, Gandy S (2013) Acute and Chronic Traumatic Encephalopathies: Pathogenesis and Biomarkers. Nat Rev Neurol 9(4): 192-200.
- Sayed N, Culver C, Dams-Oconnor K, Hammond F, Diaz-Arrastia R (2013) Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma 30(13): 1117-1122.
- Barcikowska M, Wisniewski HM, Bancher C, Grundke-Iqbal I (1989) About The Presence Of Paired Helical Filaments in Dystrophic Neurites Participating in The Plaque Formation. Acta Neuropathol 78(3): 225-231.
- Binder LI, Frankfurter A, Rebhun LI (1985) The Distribution of Tau in the Mammalian Central Nervous System. J Cell Biol 101(4): 1371-1378.
- Bugiani O, Giaccone G, Frangione B, Ghetti B, Tagliavini F (1989) Alzheimer Patients: Peamyloid Deposits are More Widely Distributed than Senile Plaques Throughout the Central Nervous System. Neurosci Lett 103(3): 263-268.
- Wisniewski HM, Narang HK, Corsellis JAN, Terry RD (1976) Ultrastructural Studies of the Neuropil & Neurofibrillary Tangles in Alzheimerʼs Disease & Post-Traumatic Dementia: 136. Journal of Neuropathology and Experimental Neurology 35(3): 367.
- Mackenzie IRA (1994) Senile plaques do not progressively accumulate with normal aging. Acta Neuropathol 87(5): 520-525.
- Ferrer I (2012) Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol 97(1): 38-51.
- Wisniewski HM, Ghetti B, Terry RD (1973) Neuritic (Senile) Plaques and Filamentous Changes in Aged Rhesus Monkeys. J Neuropathol Exp Neurol 32(4): 566-584.
- Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Götz J (2003) β-Amyloid Induces Paired Helical Filament-like Tau Filaments in Tissue Culture. J Biol Chem 278(41): 40162-40168.
- Fyfe I (2016) Dementia: Shape asymmetry of brain structures predicts dementia. Nat Rev Neurol 12(12): 679-679.
- Shaikh AG, Palla A, Marti S, Olasagasti I, Optican LM, et al. (2013) Role of Cerebellum in Motion Perception and Vestibulo-ocular Reflex—Similarities and Disparities. Cerebellum 12(1): 97-107.
- Canto CB, Onuki Y, Bruinsma B, van der Werf YD, De Zeeuw CI (2017) The Sleeping Cerebellum. Trends Neurosci 40(5): 309-323.
-
Stanislav Kolpakov Nikitin*, Arseniy Yashkin and Igor Akushevich. Differences in the Distribution of Aβ in the Brain between U.S. Veterans and Adults aged 62+ and suffering from Alzheimer’s Disease. Annal Biostat & Biomed Appli. 6(1): 2024. ABBA.MS.ID.000630.
Alzheimer’s disease neuroimaging initiative; traumatic brain injury; post-traumatic stress disorder; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






