 Research Article
Research Article
Developments and Applications of Biostatistical Time Series: A Review
Shelton Peiris*1 and Tim Swartz2
1Visiting from School of Mathematics and Statistics, The University of Sydney, Australia
2Department of Statistics and Actuarial Science, Simon Fraser University, Canada
Shelton Peiri, Visiting from School of Mathematics and Statistics, The University of Sydney, NSW, Australia.
Received Date: November 27, 2019 Published Date: December 16, 2019
Abstract
Many data sets from medical science are available as time series, especially the records in births, epidemiology, fatal accidents, medical expenses etc, where the information are collected over time. This paper gives a short review of time series methods which have been used in medical research.
Keywords: Time series; Autoregression; Serial correlation; Stationarity; ARMA; Forecasting
Introduction
A time series is a collection of measurements recorded through a suitable time scale. Although this time scale may not be equally spaced, many applications are based on equally spaced time series data. It is known that time series data are generated when a population or an important phenomenon is monitored over time. Many time series analysts use the family of autoregressive integrated moving average (ARIMA) as it enjoys fruitful applications in modelling and forecasting of time series data (ie. serially or autocorrelated data) arise in almost all social sciences. Each member of this family assumes that future values of the series have a clearly defined dynamic parametric relationship which involves both current and past values together with a random noise. A number of extensions to this ARIMA family have been developed to model time series data not following the standard assumptions. A reason for this is that accurate modelling and analysis are useful in practice to estimate potential future observations or forecast values.
Many countries around the world use time series methodology to increase the quality of human life in health, epidemiology, national planning, controlling mortality etc as they a ect their developments. Planning and forecasting of population or migration are also essential for allocation of funds for social services of nations. Therefore, developing good time series models to best suits data sets and use them for accurate forecasting is essential in applications. This review paper is dedicated and focused on a short review of modeling and forecasting through popular time series models highlighting some potential applications through the R statistical software package.
Popular Time Series Models in Practice
Suppose that we have observed a time series of n observations on { Xt } following
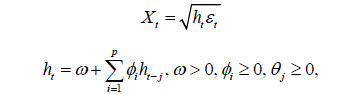
where L is the lag operator such that 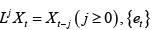 is a
sequence of uncorrelated random variables,
is a
sequence of uncorrelated random variables,  is a polynomial of order p and
is a polynomial of order p and
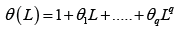 polynomial of order q: Since Xt has integrated at order d; (2.1) is known as an autoregressive integrated moving average of order ( p, d,q) and is abbreviated by ARIMA( p, d,q) : Although it isexpressed in a very general form, in many applications, we need
integers of ( p, d,q) such that 0 ≤ p, d,q ≤ 2 , and making ieasy to
apply in practice. Stationarity of the series and the orders p; d; q can
be identified through the time series plot, autocorrelation (acf) and
the partial autocorrelation (pacf) of the data. The time series plot
tells whether it needs prior transformations and/or differencing to
make it to a stationary series { Y t }Some possible models are:
polynomial of order q: Since Xt has integrated at order d; (2.1) is known as an autoregressive integrated moving average of order ( p, d,q) and is abbreviated by ARIMA( p, d,q) : Although it isexpressed in a very general form, in many applications, we need
integers of ( p, d,q) such that 0 ≤ p, d,q ≤ 2 , and making ieasy to
apply in practice. Stationarity of the series and the orders p; d; q can
be identified through the time series plot, autocorrelation (acf) and
the partial autocorrelation (pacf) of the data. The time series plot
tells whether it needs prior transformations and/or differencing to
make it to a stationary series { Y t }Some possible models are:
• If the acf of { Yt } (transformed series) or{ Xt } (original series) decays very quickly (exponentially), then the time series is called ‘short memory’ and use the standard ARMA modelling techniques to and the locallybest possible model.
• If the acf of { Yt } and{ Xt } decays very slowly (hyperbolically), then the time series is called ‘long memory’ and use the theory of fractional differencing together ARMA techniques to and the best possible model. The corresponding model is similar to that of (2.1) with a fractional degree of differencing 0 < d < 0:5: This family is known as autoregressive fractionally integrated moving average or ARFIMA(p,d,q).
Replacing the operator 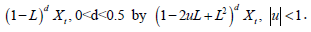 there is a very
general long memory time series known as Gegenbauer
ARMA or GARMA(p,d,q; u) with many applications
infinance and biostatistics. See for example, Dissanayake
et al. (2018) and references therein.
there is a very
general long memory time series known as Gegenbauer
ARMA or GARMA(p,d,q; u) with many applications
infinance and biostatistics. See for example, Dissanayake
et al. (2018) and references therein.
• For certain time series data sets, the acf is not significant for the original data on t X but the acf of 2 t X is very significant. Such data can be modelled using the theory on generalized autoregressive conditional heteroscedastic (GARCH) models. This family of GARCH (p; q) is given by
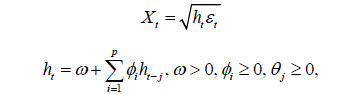
Where 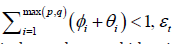
comprises of a stationary sequence of independent and identically
distributed random variables satisfying zero mean, unit
variance and ht is the conditional variance of { Xt } given the history;
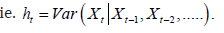
Illustrations
Below we illustrate these three cases in applications. Notice that acf of the last plot shows no serial correlation of data, however, Data2 (or Data2) contains significant acf values (Figure 1).
Now we look at an application of ARIMA modelling using a biostatistical time series data set.

ARIMA Modelling in Biostatistics
This section considers an application from annual incidences of stomach cancer in Australia from 1982 to 2015 (available in Cancer data in Australia https://www.aihw.gov.au/) to illustrate the procedure. The time series plot of data shows a slight upward trend. We conduct the analysis based on both the original and lag 1 differenced data and select the best possible model from a pool of all potential models. Time series plots of cancer cases (original data) and Differenced data (lag one differenced data) together with corresponding acf and partial pacf are given below: (Figure 2).
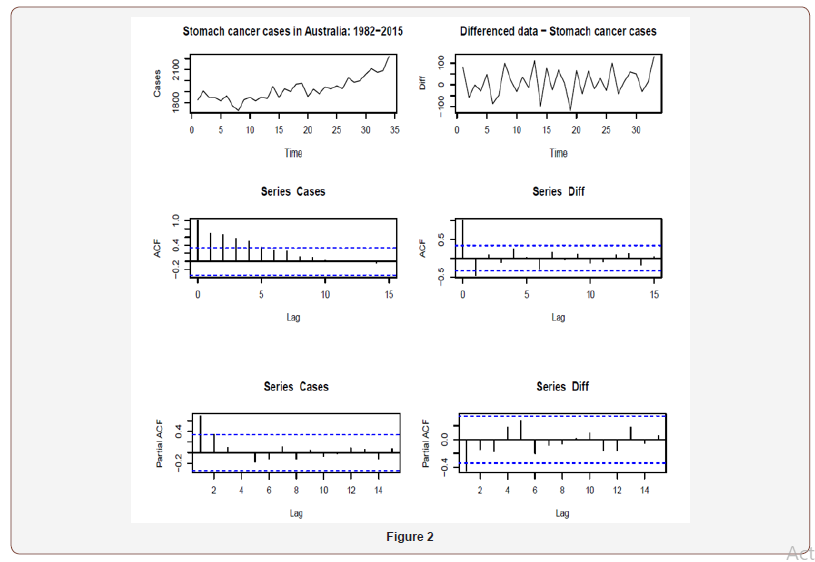
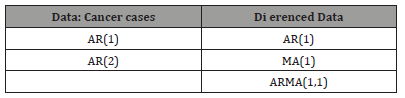
Based on the above plots, suggest the following ARMA models: Table 1
Table 1:
Estimating each model using R give the following summary (values in parentheses are the corresponding estimated standard errors) Table 2:
Table 2:
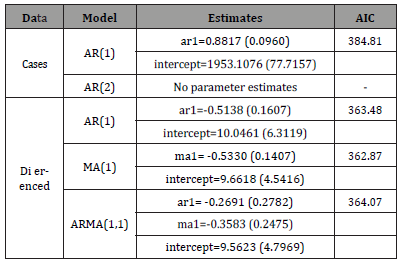
This tells us that the differenced MA(1) model for the differenced
data is the best among those have been suggested since it has the
smallest aic value. Let { Xt } be the original series and
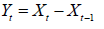 represents the differenced series. Therefore, the best fitted model
for { Yt } is
represents the differenced series. Therefore, the best fitted model
for { Yt } is

where {et} is the associated noise.
Further applications of ARIMA modelling in biostatistical time series can be found in Cox and Solomon (1988), Zeger et al. (2004), Zeger et al. (2004), Perera et al. (2008), Zollar et al. (2016), Siskina and Siaulys (2016), Yousefzadeh-Chabok et al. (2016), Ferenci (2017), Fried et al. (2017), Xie (2017), Pavareh et al. (2018),Dissanayake et al. (2018), Earnest (2019) and references theirin.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Mojab F, Poursaeed M, Mehrgan H, Pakdaman S (2008) Antibacterial activity of Thymus daenensis methanolic extract. Pak J Pharm Sci 21(3): 210-213.
- Hancock EW (2005) Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis 5(4): 209-218.
- Nagesh KS, Shanthamma C (2009) Antibacterial activity of Curculigo orchioides rhizome extract on pathogenic bacteria. African Journal of Microbiology Research 3(1): 005-009.
- Jayaprakasha GK, Selvi T, Sakariah KK (2003) Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Research International 36(2): 117-122.
- Singh RP, Murthy KNC, Jayaprakasha GK (2002) Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 50(1): 81-86.
- Kabuki T, Nakajima H, Arai M, Ueda S, Kuwabara Y, et al. (2000) Characterization of novel antimicrobial compounds from mango (Mangifera indica L) kernel seeds. Food Chemistry 71(1): 61-66.
- Prasad KN, Xie H, Hao J, Yang B, Qiu S, et al. (2010) Antioxidant and anticancer activities of 8 – hydroxypsoralen isolated from wampee (Clausena lansium Lour. Skeels) peel. Food Chemistry 118(1): 62-66.
- Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S (2009) Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India. Indian J Pharm Sci 71(4): 406-412.
- Boyer J, Liu R (2004) Apple phytochemicals and their health benefits. Journal of Nutrition 3: 5.
- Potter D, Eriksson T, Evans RC, Oh SH, Smedmark JEE, et al. (2007) Phylogeny and classification of Rosaceae. Plant Systematics and Evolution 266(1-2): 5-43.
- Pastene E, Speisky H, Troncoso M, Alarcon J, Figueroa G (2009) In vitro inhibitory effect of apple peel extract on the growth of Helicobacter pylori and respiratory burst induced on human neutrophils. J Agric Food Chem 57(17): 7743-7749.
- Suarez B, Alvarez AL, Garcia YD, Barrio G, Lobo AP, et al. (2010) Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chemistry 120(1): 339-342.
- Garcia YD, Valles BS, Lobo AP (2009) Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chemistry 117(4): 731-738.
- Zessner H, Pan L, Will F, Klimo K, Knauft J, et al. (2008) Fractionation of polyphenol enriched apple juice extracts to identify constituents with cancer chemopreventive potential. Mol Nutr Food Res 52(Suppl 1): 128-144.
- Rupasinghe HPV (2003) Using change for success: Fruit-based bioproduct research at the Nova Scotia Agricultural College. Annual Report 2003 of the Nova Scotia Fruit Growers Association 66-69.
- Bhushan S, Kalia K, Sharma M, Singh B, Ahuja PS (2008) Processing of apple pomace for bioactive molecules. Crit Rev Biotechnol 28(4): 285-296.
- Alberto MR, Gómez Cordovés C, Manca De Nadra MC (2004) Metabolism of gallic acid and catechin by Lactobacillus hilgardii from wine. J Agric Food Chem 52(21): 6465-6469.
- Malaviya A, Mishra N (2011) Antimicrobial activity of tropical fruits. International Journal of Biology 3(1): 1-4.
- Muthuswamy, Rupasinghe HPV (2007) Fruit phenolics as natural antimicrobial agents: Selective antimicrobial activity of catechin, chlorogenic acid and phloridzin. Journal of food agriculture & environment 5(3-4): 81-85.
- Jelodarian S, Ebrahimabadi AH, Kashi JF (2013) Evaluation of antimicrobial activity of Malus domestica fruit extract from Kashan area. Avicenna J Phytomed 3(1): 1-6.
- Sadeghian A, Ghorbani A, Mohamadi Nejad A, Rakhshandeh H (2011) Antimicrobial activity of aqueous and methanolic extracts of pomegranate fruit skin. Avicenna Journal of Phytomedicine 1(2): 67-73.
- Spigno G, Tramelli L, De Faveri DM (2007) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. Journal of Food Engineering 81(1): 200-208.
- Dent M, Dragović Uzelac V, Penic M, Brncic M, Bosiljkov T, et al. (2013) The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols of dalmatian wild sage (Salvia officinalis L.) Extracts. Food Technology and Biotechnology 51(1): 84-91.
- Hiba N, Rajha Darra EN, Vorobiev E, Louka N, Richard G, et al. (2013) An environment friendly, low-cost extraction process of phenolic compounds from grape Byproducts. Optimization by multi-response surface methodology. Food and Nutrition Science 4(6): 650-659.
- Pinelo M, Rubilar M, Sineiro J, Nunez MJ (2004) Extraction of antioxidant phenolics from almond hulls (Prunus Amygdalus) and pine sawdust (Pinus pinaster). Food Chemistry 85(2): 267-273.
- Wissam Z, Ghada B, Wassim A, Warid K (2012) Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. International Journal of Pharmacy and Pharmaceutical Sciences 4(suppl 3): 675-682.
- Muralidhar RV, Chirumamila RR, Marchant R, Nigam P (2001) A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochemical Engineering Journal 9(1): 17-23.
- Kelmanson JE, Jager AK, Van SJ (2000) Zulu medicinal plants with antibacterial activity. J Ethnopharmacol 69(3): 241-246.
- Parikh P, McDaniel MC, Ashen MD, Miller JI, Sorrentino M, et al. (2005) Diets and cardiovascular disease. An evidence-based assessment. J Am Coll Cardiol 45(9): 1379-1387.
- Holley AR, Patel D (2005) Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiology 22: 273-292.
- Fratianni F, Sada A, Cipriano L, Masucci A, Nazzaro F (2007) Biochemical characteristics, antimicrobial and mutagenic activity in organically and conventionally produced Malus domestica, Annurca. The Open Food Science Journal 1: 10-16.
- Jeong MR, Kim HY, Jeong Dan CJD (2009) Antimicrobial activity of methanol extract from Ficus carica leaves against oral bacteria. Journal of Bacteriology and Virology 39(2): 97-102.
- Balakrishnan N, Bhaskar VH, Jayakar B, Sangameswaran B (2006) Short communication antibacterial activity of Mimosa pudica, Aegle marmelos and Sida carlifolia. Pharmcognosy Magazine 2(7): 198-199.
-
Shelton Peiris, Tim Swartz. Developments and Applications of Biostatistical Time Series: A Review. Annal Biostat & Biomed Appli. 3(5): 2019. ABBA.MS.ID.000571.
Time series, Autoregression, Serial correlation, Stationarity, ARMA, Forecasting
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






