 Research Article
Research Article
Physical Model for the effect of Weak Alternating Electric Fields on Bacterial Growth Rate and Viability
Amr Aboushousha1*, Hans-Georg Breitinger2, Ulrike Breitinger2 and Ali Mubarak3
1Physics department, Faculty of Basic science, German university in Cairo, Physics department, Faculty of science, Cairo university, Egypt
2Biochemistry department, Faculty of Pharmacy and Biotechnology, German university in Cairo, Egypt
3Biophysics institute, Ulm university, Germany
Amr Aboushousha, Physics department, Faculty of Basic science, German university in Cairo, Physics department, Faculty of science, Cairo university, Egypt
Received Date: February 12, 2025; Published Date: March 11, 2025
Abstract
Keywords:Alternating electric fields; bacterial growth rate; physical model of electric fields action on bacterial membranes
Introduction
Multidrug resistant bacterial infections impose a great danger to humanity that is sadly inevitable due to the widespread use of antibiotics [1] this in turn requires novel methods treating bacterial infections rather than the pharmacochemical approach, physical approaches are promising candidates to be used as valid and applicable treatment methods against bacterial infections. Physical methods include Photodynamic therapy [2-4] ultrasound wave therapy [5-7] thermotherapy [8] weak electric currents [9,10] and recently high frequency alternating electric fields [11,12] the downside of the first four methods being: 1-low therapeutic index relative to the high heating produced by photodynamic therapy, ultrasound wave therapy and thermotherapy [13], 2-activated oxygen species resulting from photodynamic therapy treatment [14]. 3- toxic ions as a result of the use of conductive electrodes [15] also the fact that none of these methods ever matured into an approved treatment method. As for electric currents, experiments focused on viability, metabolism and transport in cells affected by currents [16,17] despite the discovery of the bioelectric phenomena which is synergy between a relatively weak electric current in combination with an antibiotic that would eradicate biofilm bacteria [18].
It was observed that electric currents even the weakest of them had four major drawbacks 1- nerve stimulation and muscle contraction, 2- inability to apply electric currents to deep lesions, 3- inability to apply electric currents using isolated electrodes, 4-the need for long durations for DC in order to have an effect [11]. On the other side it is a different situation for alternating electric fields as it was shown that there is a spectrum of electric fields called anti- microbial fields having no harming effect on human cells, which ranges from (5-30) MHz optimally 10 MHz, which makes alternating electric fields an applicable approach for treating bacterial infections [12]. The factors that govern the alternating electric field effect on bacterial cells are of wide variety being field frequency and intensity , duration of field application , the medium that bacterial cells grow in and the change in temperature due to the application of the electric field [19-22] knowing the factors affecting the electric field effect on bacterial cells there are still two main concepts to put in mind , AM Fields (Anti-microbial fields) being alternating electric field with a range of frequency between(5-30) MHz optimally used at 10 MHz allowing the use of relatively high field intensities [12].
The second concept being the ability to use lower field intensities over longer time and producing the same effect on bacterial cells [19]. Escherichia coli k-12 was the bacterial species of choice for two major reasons the first being well defined genetically, metabolically and structurally and the second being that E-coli is a gram negative bacteria which in turn means that it has an outer membrane with 10% of it negatively charged which can be a target for alternating electric fields effects, the fact that the outer membrane of gram negative bacteria has proteins impeded into it also being targets for alternating electric fields [22-24]. In these experiments we are targeting E-coli membrane proteins specifically outer-membrane proteins using alternating electric fields which in turn affected bacterial cell growth rate, then explaining the results which are based only on the electric field effect only without the intervening of heat or the use of antibiotics via a semi-classical physical model that can be applied to all membrane proteins.
Material and Methods
Bacterial strain and growth conditions: E-coli strain K-12 was from the former Namru-3 unit in Egypt. Bacteria were grown in LB medium with the composition of (10gm NaCl, 10gm Triptone and 5gm Yeast extract per liter). Broth culture of freshly plated bacteria was grown in 250 ml of liquid medium at 37C0 in an orbital shaker up to logarithmic phase, and then bacterial stocks were taken from the culture and diluted to the starting OD600 of all samples in each experiment. AM Fields generation system: the fields were emitted by a pair of 50cm x 50cm parallel metallic plates with no contact with the bacterial medium, the plates separated by 1.5 cm high; resulting a field strength of (1333v/m), the electric field was generated by a signal generator (Koolerton CJD S66 series digital control dual channel DDS signal generator REV.1).
The OD600 measurements done to measure the difference in bacterial growth rates were obtained using single beam spectrophotometers at timely intervals. Experiments were done in 25C0 and there was no heat induction by the metallic plates covered in electric insulators, bacteria were cultured in LB medium which is a favored medium for E. coli growth, this setting has led to the results only being based on the electric field bacteriostatic effect only without the addition of any other bacteriocidal effect such as heating, antibiotics or immune system interference as in living organisms. Bacterial plates were in sets of 3 with a set of controls away from the electric fields, all plates were shaken at a rate of 3 cycles per minute to allow good aeration and keep equal distribution of bacterial cells in liquid cultures (Figures 1a-1c).
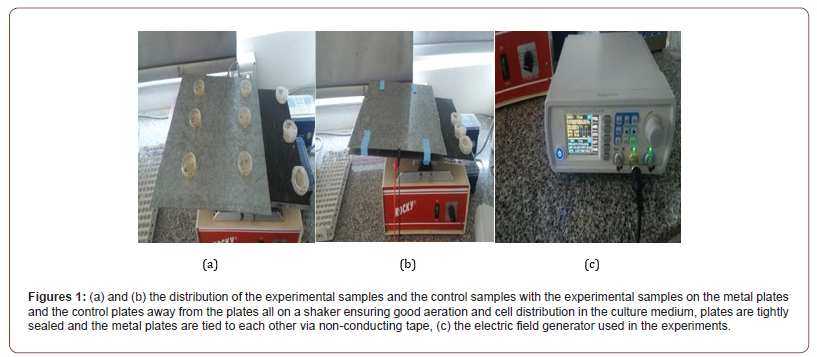
Results
Short Exposure Period Experiment
In this experiment the electric field parameters were V= 20 V, f= 10 MHz and durations of exposure of 30 minutes, with the starting cell count to be 114.38 x 106 CFU/ml. The results are shown in the following (Figures 2&3).
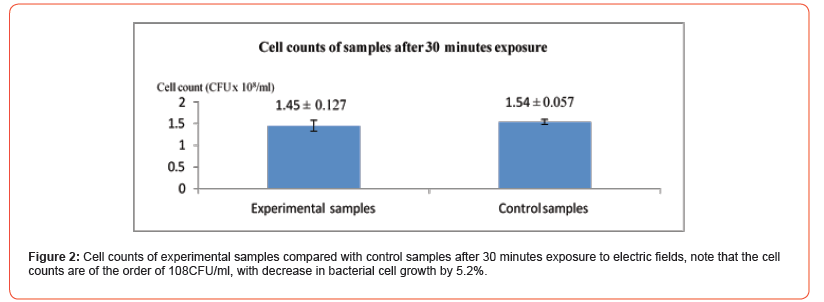
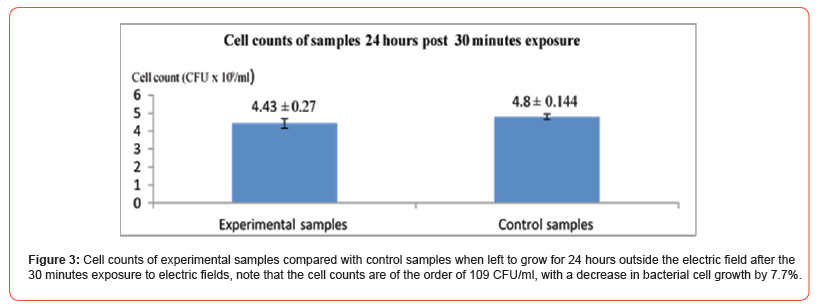
Long Exposure Period Experiment
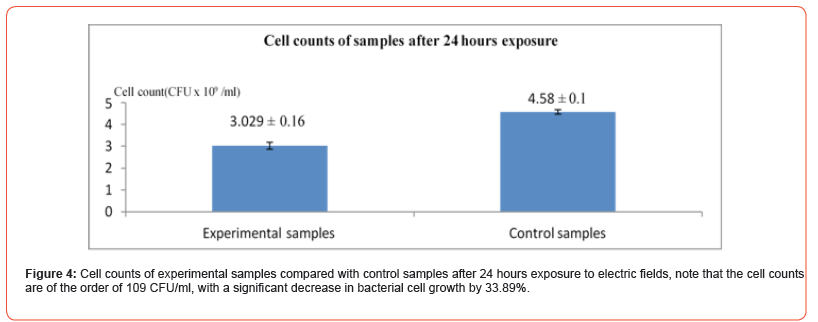
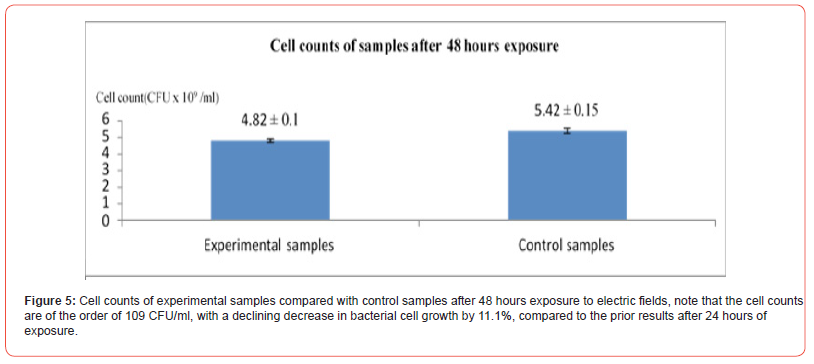
In this experiment we used field parameters of V= 20V, f = 10MHz and duration of exposure of 24 and 48 hours, in this experiment all samples had a starting cell count of 34.58 x 106 CFU/ml (Figure 4&5). The results of this experiment show that the electric field effect vary with increasing the duration of exposure though the potential was not critical there is an obvious 33.89% decrease in bacterial growth at the 24 hours interval, but still after some time bacterial cells started to catch up in terms of growth rates nearing the plateau phase due to competition for nutrients.
Discussion
Microbiological Model
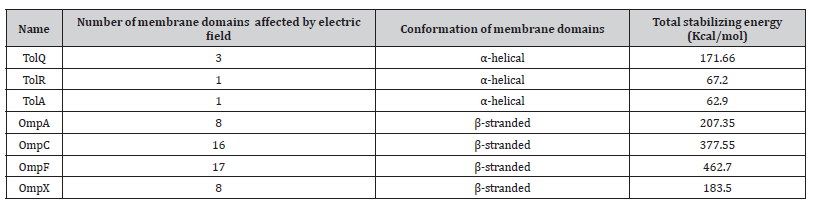
In this research we observed the effect of alternating electric fields in isolation of any other factor on bacterial growth rates and we propose a model that serves as an explanation to the effect measured during these experiments, this model is applied to membrane proteins that possibly relate to the bacteriostatic effect of electric fields besides being well studied, four of them being outer membrane porins (OmpA, OmpC, OmpF and OmpX) and three being inner membrane proteins belonging to the Tol-Pal system (TolQ, TolR and TolA), the effect on previously mentioned proteins will be discussed in the following section.
Tol-Pal System
Tol-Pal system is an envelope spanning five-protein complex present in gram-negative bacteria composed of three inner membrane protein (TolQ, TolR and TolA) one periplasmic protein (TolB) and a peptidoglycan associated protein (Pal), coded in the Ybge, TolQ, TolR, TolA, Pal, Cpob gene cluster [25-27]. Interactions of Tol- Pal proteins result in linking the inner and outer membranes capable of energy transduction. TolQ and TolR share structural homology with ExbB and ExbD of the TonB system and also with MotA and MotB of the flagellar motor system as well as functional homology as all of these systems are driven by proton motive force [27-29]. As for the proton motive needed in the Tol-pal system it is elucidated that TolQ and TolR form a channel for proton movement to drive the interaction between TolA and Pal [30]. Observations that Tolpal mutants are unable to undergo cell division leading to forming of cell chains, combined with the results showing that disruption of the outer membrane leads to remarkably increased susceptibility to some antimicrobial agents such as β-lactams, colistin, vancomycin and novobiocin [31,32].
OmpA
OmpA functions vary from structural function as in binding to the peptidoglycan noncovalently [33] maintaining the position of the peptidoglycan cell wall in the periplasm as a result of the interaction with TolR, holding it equidistant from the inner and outer membranes [34,35] and enabling resistance to environmental stress and maintains the functional and structural integrity of the outer membrane [36,37] to regulatory function as a porin with low permeability allowing slow passage of small solutes [38-40]. Disruption of OmpA causes loss in outer membrane integrity and causes weaker stress survival [36] as well as disruption of TolR which causes the Tol-Pal system disruption leading to even more instability to gram negative cells.
OmpC and OmpF
Form pores that allow passive diffusion of small molecules across the outer membrane [41,42] in turn disrupting this function may lead to osmotic imbalance which can hinder bacterial growth.
OmpX
Having an exposed beta sheet edge, it is proposed to function as “fishing rod” that binds with complementary beta strands at their surface, this binding affinity is suspected to be used for cell adhesion and invasion and to interfere with human compliment defense system which in turn causes virulence [43] although disrupting such protein may not cause lowering bacterial growth rate but rather the virulence of gram-negative bacteria.
Energy Estimate of Intra-Membrane Domain Interactions Stabilizing a Membrane Protein
Estimate of Side Chain Interactions done by Each Amino acid in An Intra-Membrane Domain
Estimating the energetics of interactions holding an intra-membrane domain in a membrane is based on the accumulation of many weak side chain interactions possible to occur between the amino acid side chain and the membrane which include hydrogen bonding, hydrophobic interactions majorly [44]. The numbers used to estimate the energies of the interaction are based on previous studies targeting each interaction specifically. For side chain hydrogen bonds, it is estimated to contribute to membrane domain stability by 1.1±0.8 Kcal/mol, hydrophobic interactions on the other hand contribute to the stability of a protein with 1.1±0.5 Kcal/mol per (-CH2-) in the side chain in case of aliphatic hydrophobic amino acids [45], in case of aromatic amino acids contributions to protein stability on average are 4.5 Kcal/mol for Tryptophan, 4.65 Kcal/ mol for Tyrosine and 3.4 Kcal/mol for Phenylalanine [46]. Note that Cysteine contribution is not included with other amino acids as it contributes with disulfide bonds as the estimate of this bond strength, ΔS, is calculated by this equation:
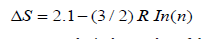
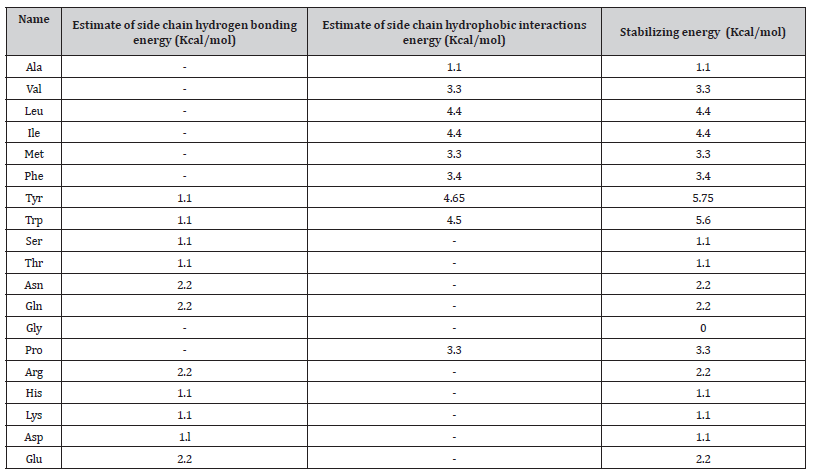
As R is the gas constant and n is the number of the amino acids in the loop formed by the crosslink (44), which is calculated in a case-by-case manner. Possible hydrogen bonding side chain atoms that can interact with the bacterial membrane are those capable of accepting hydrogen bonds forming weak hydrogen bonds (C-H…. O and C-H…. N) [47] (Table 1).
Table 1:Estimates of side chain interactions stabilizing an intra-membrane domain.
Key Information Regarding Membrane Proteins Studied Essential to The Physics Model
Table 2:Main data regarding studied membrane proteins.
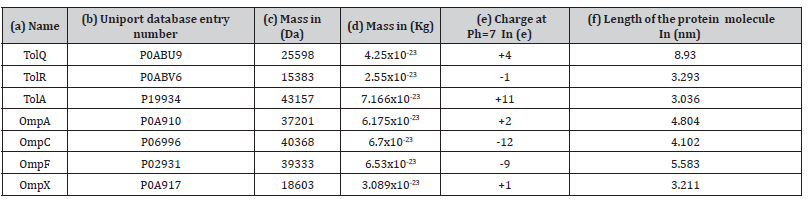
(a) Protein name abbreviations, (b) Uniprot database entry number for each protein, (c) Mass of the protein in Daltons, (d) Mass of the protein
converted from Daltons to Kilograms (1Da =1.66053907×10−27Kg). (e) Charge of the protein in terms of electron charge at Ph = 7 , calculated using the following formula: Charge 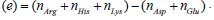 . (f) length of the protein in (nm) calculated from high resolution 3d structures found in uniport database.
. (f) length of the protein in (nm) calculated from high resolution 3d structures found in uniport database.
The Physics Model
Our explanation of the results is based on a semi-classical description
of membrane proteins behavior when exposed to an oscillating
electric field; the protein is approximated to a point charge 𝑞
that has the value of the collective charge on the protein. The protein
has a mass 𝑚 and is embedded in the membrane through its thickness with a length of 3nm in case of the inner membrane and
2.5 nm in case of the outer membrane [48,49]. The stabilizing energy
of different membrane proteins introduced in Table 3.1 holds the
protein in the membrane. When a charge 𝑞 is affected by a sinusoidal
oscillating electric field of magnitude E that is generated by an
oscillating electric potential difference ΔV , the charge gains energy
of qΔV [50]. This energy will set the charge into mechanical oscillation
with vibrational energy of 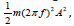 , where 𝑚 is the mass of the oscillating particle (protein), 𝑓 is the frequency of the applied potential difference (field) and 𝐴 is the amplitude of motion.
, where 𝑚 is the mass of the oscillating particle (protein), 𝑓 is the frequency of the applied potential difference (field) and 𝐴 is the amplitude of motion.
Table 3.1:Calculation of the intra-membrane domain stabilizing energy for each of the protein under study.
In table 3 we show our calculation for the oscillation’s amplitude for different proteins in such an oscillating field:
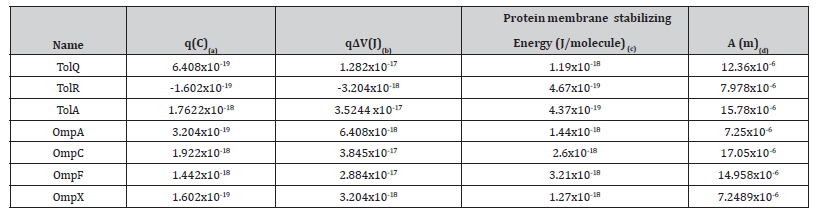
Table 3.2:main calculations needed for the physical model explaining the bacteriostatic effect of electric fields: (a) Charge converted from electron charge to coulombs using the following relation: e =1.602×10−19C , (b) Charge of the protein multiplied by the potential difference between the metallic plates generating the electric fields, (c) Estimate of energy stabilizing the membrane incorporated parts of the protein within the membrane in joules converted from Kcal/mol to J/molecule using the following relation:
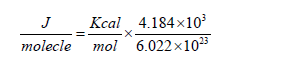
(d) Amplitude of the vibration of the membrane proteins induced by the electric fields in (m).
Equating the mechanical energy of vibrations with that gained from the oscillating electric potential (field) we get:
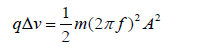
The amplitude of oscillations becomes then:
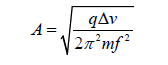
In Table 3.2 we show our calculation for the oscillation’s amplitude for different proteins in such an oscillating field.
One can easily recognize that all the amplitudes are much longer compared to the membrane thickness by a factor of 100. Another important finding is that the energy gained by a protein from the oscillating potential difference is at least 10 times bigger than the stabilizing energy of the membrane protein required to keep that protein stable in the membrane. Both calculations above led us to assume that the protein will be snatched out of the membrane when exposed to such an oscillating electric field. Hence that protein will not be able to perform its functions in maintaining the structural and functional integrity of the bacterial membrane. Of course, that will act against the survival of the bacterial cell and its subsequent division (growth). That physical action reflected itself on the inhibition of the bacterial growth in the samples exposed to the alternating electric field at different durations. It continued however till 24 hours after stopping the field.
Conclusion and Future Work
The introduced Physical model explains the inhibition of the growth of the E-Coli bacteria by using the microbiological description of the outer membrane porin proteins and the Tol- Pal membrane incorporated proteins. This was achieved by considering the protein a charged particle set into driven large amplitude oscillations by a low intensity, alternating, high frequency electric field. The energy of oscillations provided by the field was found to be much greater than the stabilizing energy provided by the protein-membrane system. Such proteins were snatched out of the bacterial membrane. Such process will reduce the vitality and survival of bacterial cells. As promising future extension of our research, we want to test the process on different type of bacteria especially those that are antibiotic resistance and examine the subsequent effect of adding an antibiotic after such exposure to an oscillating electric field. We believe that this method introduces a solution to the antibiotic resistance that is appearing an increasing manner.
References
- Dzidic S, Suskovic J, Kos B (2008) Antibiotic resistance mechanisms in bacteria: biochemical and genetic aspects, Food Technol Biotechnol 46(1): 11-21.
- Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, et al. (2006) Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med 38(5): 468-481.
- Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 22(2): 83-91.
- Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42(1): 13-28.
- Ensing GT, Roeder BL, Nelson JL, van Horn JR, van der Mei HC, et al. (2005) Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J Appl Microbiol 99(3): 443-448.
- Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD (1994) Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob Agents Chemother 38(11): 2577-2582.
- Rediske AM, Rapoport N, Pitt WG (1999) Reducing bacterial resistance to antibiotics with ultrasound. Lett Appl Microbiol 28(1): 81-84.
- Reithinger R, Mohsen M, Wahid M, Bismullah M, Quinnell RJ, et al. (2005) Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled Clin Infect Dis 40(8): 1148-1155.
- Caubet R, Pedarros-Caubet F, Chu M, Freye E, de Belem Rodrigues M, et al. (2004) A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob Agents Chemother 48(12): 4662-4664.
- Del Pozo JL, Rouse MS, Euba G, Kang CI, Mandrekar JN, et al. (2009) The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother 53(10): 4064-4068.
- Giladi M, Porat Y, Blatt A, Wasserman Y, Kirson ED, et al. (2008) Microbial growth inhibition by alternating electric fields. Antimicrob Agents Chemother 52(10): 3517-3522.
- Giladi M, Porat Y, Blatt A, Shmueli E, Wasserman Y, et al. (2010) Microbial growth inhibition by alternating electric fields in mice with Pseudomonas aeruginosa lung infection. Antimicrob Agents Chemother 54(8): 3212-3218.
- Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, et al. (2009) In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol 9: 27.
- Oleinick NL, Evans HH (1998) The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res 150(5): S146-S156.
- Liu WK, Brown MR, Elliott TS (1997) Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 39(6): 687-695.
- Pareilleux A, Sicard N (1970) Lethal effects of electric current on Escherichia coli. Appl Microbiol 19(3): 421-424.
- Rosenberg B, Vancamp L, Krigas T (1965) Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205: 698-699.
- Sandvik EL, Mcleod BR, Parker AE, Stewart PS (2013) Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid, PLoS One 8(2): e55118.
- Shawki MM, Gaballah A (2015) The effect of low AC electric field on bacterial cell death. Rom J Biophys 25(2): 163-172.
- Hülsheger H, Potel J, Niemann EG (1983) Electric field effects on bacteria and yeast cells. Radiat Environ Biophys 22(2): 149-62.
- Kinosita K, Tsong TY (1979) Voltage-induced conductance in human erythrocyte membranes. Biochim Biophys Acta 554(2): 479-497.
- Idalia Vargas-Maya, Franco, Bernardo (2017) Escherichia coli as a Model Organism and Its Application in Biotechnology.
- Wang J, Ma W, Wang X (2021) Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb Cell Fact.
- Joel HW, Liang Li (2008) Proteome of the Escherichia coli envelope and technological challenges in membrane proteome analysis. Biochim Biophys Acta 1778(9): 1698-1713.
- Lloubès R, Cascales E, Walburger A, Bouveret E, Lazdunski C, et al. (2001) The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane Res Microbiol 152(6): 523-529.
- Vianney A, Muller MM, Clavel T, Lazzaroni JC, Portalier R, et al. (1996) Characterization of the tol-pal region of Escherichia coli K-12: translational control of TolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J Bacteriol 178(14): 4031-4038.
- Cascales E, Lloubès R, Sturgis JN (2001) The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB. Mol Microbiol 42(3): 795-807.
- Cascales E, Gavioli M, Sturgis JN, Lloubès R (2000) Proton motive force drives the interaction of the inner membrane TolA and outer membrane pal proteins in Escherichia coli. Mol Microbiol 38(4): 904-915.
- Goemaere EL, Cascales E, Lloubès R (2007) Mutational analyses define helix organization and key residues of a bacterial membrane energy-transducing J Mol Biol 366(5): 1424-1436.
- Gerding MA, Ogata Y, Pecora ND, Niki H, de Boer PA (2007) The trans- envelope Tol-Pal complex is part of the cell division machinery and required for proper outer- membrane invagination during cell constriction in coli. Mol Microbiol 63(4): 1008-1025.
- Yakhnina AA, Bernhardt TG (2020) The Tol-Pal system is required for peptidoglycan-cleaving enzymes to complete bacterial cell Proceedings of the National Academy of Sciences 117(12): 201919267.
- Hirakawa H, Suzue K, Tomita H (2022) Roles of the Tol/Pal System in Bacterial Pathogenesis and Its Application to Antibacterial Therapy. Vaccines 10(3): 422.
- Ishida H, Garcia-Herrero A, Vogel HJ (2014) The periplasmic domain of Escherichia coli outer membrane protein A can undergo a localized temperature dependent structural transition. Biochim Biophys Acta 1838(12): 3014-3024.
- Boags AT, Samsudin F, Khalid S (2019) Binding from Both Sides: TolR and Full- Length OmpA Bind and Maintain the Local Structure of the E. coli Cell Wall. Structure 27(4): 713-724.e2.
- Samsudin F, Ortiz-Suarez ML, Piggot TJ, Bond PJ, Khalid S (2016) OmpA: A Flexible Clamp for Bacterial Cell Wall Structure 24(12): 2227-2235.
- Wang Y (2002) The function of OmpA in Escherichia coli. Biochem Biophys Res Commun 292(2): 396-401.
- Sonntag I, Schwarz H, Hirota Y, Henning U (1978) Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol 136(1): 280-285.
- Ishida H, Garcia-Herrero A, Vogel HJ (2014) The periplasmic domain of Escherichia coli outer membrane protein A can undergo a localized temperature dependent structural transition. Biochim Biophys Acta 1838(12): 3014-3024.
- Sugawara E, Nikaido H (1992) Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem 267(4): 2507-2511.
- Negoda A, Negoda E, Reusch RN (2010) Oligo-(R)-3-hydroxybutyrate modification of sorting signal enables pore formation by Escherichia coli Biochim Biophys Acta 1798(8): 1480-1484.
- Liu YF, Yan JJ, Lei HY, Teng CH, Wang MC, et al. (2012) Loss of outer membrane protein C in Escherichia coli contributes to both antibiotic resistance and escaping antibody-dependent bactericidal activity. Infect Immun 80(5): 1815-
- Duval V, Nicoloff H, Levy SB (2009) Combined inactivation of lon and ycgE decreases multidrug susceptibility by reducing the amount of OmpF porin in Escherichia coli. Antimicrob Agents Chemother 53(11): 4944-4948.
- Vogt J, Schulz GE (1999) The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of Structure 7(10): 1301-1309.
- Lee AG (2003) Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 1612(1): 1-40.
- Nick Pace C, Scholtz JM, Grimsley GR (2014) Forces stabilizing FEBS lett 588(14): 2177-2184.
- Hong H, Park S, Jiménez RH, Rinehart D, Tamm LK (2007) Role of aromatic side chains in the folding and thermodynamic stability of integral membrane J Am Chem Soc 129(26): 8320-8327.
- Rowe RK, Ho PS (2017) Relationships between hydrogen bonds and halogen bonds in biological Acta Crystallogr B Struct Sci Cryst Eng Mater 73(Pt 2): 255-264.
- Pluhackova K, Horner A (2021) Native-like membrane models of coli polar lipid extract shed light on the importance of lipid composition complexity. BMC Biol 19(1): 4.
- Wu EL, Fleming PJ, Yeom MS, Widmalm G, Klauda JB, et al. (2014) coli outer membrane and interactions with OmpLA. Biophys J 106(11): 2493-2502.
- Serway R, Jewett J (2017) Physics for scientists and engineers with modern Tenth edition C.
-
Amr Aboushousha*, Hans-Georg Breitinger, Ulrike Breitinger and Ali Mubarak. Physical Model for the effect of Weak Alternating Electric Fields on Bacterial Growth Rate and Viability. Annal Biostat & Biomed Appli. 6(3): 2025. ABBA.MS.ID.000638.
Alternating electric fields; bacterial growth rate and physical model of electric fields action on bacterial membranes; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






