 Research Article
Research Article
Histamine in Some Fish Products
Hassan M, Shaltout FA* and Saqur N
Department of Food Control, Benha University, Egypt
Shaltout FA, Department of food control, Benha University, Faculty of Veterinary Medicine, Egypt.
Received Date: January 08, 2020; Published Date: February 03, 2020
Abstract
Histamine compounds are members of a group of compounds known as biogenic amines normally produced by decarboxylation of free amino acids and are present in a variety of foods. In the present study a total of 90 random samples of salted and smoked fish products represented by fesiekh, salted sardine, and smoked herring (30 of each) were collected from different fish markets in Middle Delta area, Egypt and examined for the presence of histamine by ELISA. The results recorded that the histamine mean values in examined fish samples were20.76 ± 0.54, 15.49 ± 0.31 and 9.82 ± 0.26mg/kg, unaccepted samples were53.3%, 36.7% and 30%for fesiekh, sardine and smoked herring, respectively.
Keywords: ELISA; Histamine compounds; Salted; Smoked fish
Introduction
Fish is very important source of protein especially in Egypt. Fish and fish products are one of the most important food stuffs as they are one of the cheapest sources of animal protein. Fish are enriched with essential minerals, vitamins, and unsaturated fatty acids [1]. Histamine is known as a biogenic amine which is low molecular weight and possesses biological activity [2]. The levels of histamine have been suggested as rapid fish spoilage indicators [2,3]. Gramnegative histamine producing bacteria are more common in fish. A wide range of Gram-negative bacteria can produce histamine in fish, but the major types are mesophilic enteric and marine bacteria. Morganella morganii, Morganella psychrotolerans, Photobacterium damselae, Photobacterium phosphoreum, Raoultella planticola, Hafnia alvei were reported as histamine formers. In the case of fermented sea food, Staphylococcus spp. and Tetragenococcus spp [4]. These types of bacteria naturally present on the gills, external surfaces and in the gut of live saltwater fish with no harm to the fish. Up on death, the defense mechanism of the fish no longer inhibits bacterial growth in the muscle tissue and histamine forming bacteria may start to grow resulting in the formation of biogenic amines [5].scombroid poisoning is a form of toxicity caused by the ingestion of spoiled dark-flesh fishes, mainly of the scombroid family. The clinical picture is secondary to histamine toxicity, manifest ed as flushing, headache, palpitations, and abdominal cramps [6]. Inadequate cooling following harvest promotes bacterial histamine production and can result in outbreaks of scombroid poisoning [7] which results from the ingestion of histamine-contaminated fish of the scombroid fish including tuna, mackerel, and non-scombroid fish include sardine, herring and anchovy [8].The symptoms of scombroid poisoning appear within a few minutes after eating fish of Scombridae family and related species. The first symptoms are cutaneous, with flush, pruritus, and erythema of the face and trunk having an urticarial appearance, together with faintness. Gastrointestinal symptoms include nausea, vomiting, abdominal cramps and occasionally diarrhea [9].
This work aimed to determine histamine residue in salted and smoked fish collected from different fish markets in Gharbia governorate, Egypt by using ELIZA technique to asses quality of fish.
Materials and Methods
Collection of Samples
Collection of Samples
Determination of Histamine By ELISA
Sample Preparation and Acylation
i. Pipette 25 μL of standards, 25 μL of controls, 25 μL of plasma samples, 10 μL of fish samples, or 50 μL of supernatant from the release test* into the respective wells of the Reaction Plate.
ii. 25 μL of Acylation Buffer were added to all wells.
iii. 25 μL of Acylation Reagent were added to all wells.
iv. Incubated for 45 min at RT (20-25°C) on a shaker (approx. 600 rpm).
v. 200 μL of distilled water were added to all wells.
vi. Incubated for 15 min. at RT (20-25°C) on a shaker (approx. 600 rpm).
vii. 25 μL of the prepared standards, controls, and samples were taken for the Histamine ELISA.
*For the release test the Histamine Release supplementary kit (available for purchase separately, cat. no. BA E-1100) must be used.
Histamine ELISA
i. 25 μL of the acylated standards, controls, and samples were pipetted into the appropriate wells of the Histamine Microtiter Strips.
ii. 100 μL of the Histamine Antiserum were pipetted into all wells and cover plate with adhesive foil.
iii. Incubated for 3 hours at RT (20-25°C) on a shaker (approx. 600 rpm).
Alternatively: shake the Histamine Micro titer Strips briefly by hand and incubate for 15-20 hours at 2-8°C.
i. The foil was removed. The contents of the wells were discarded or aspirated, and each well was washed 4 times thoroughly with 300 μL Wash Buffer. Blotted dry by tapping the inverted plate on absorbent material.
ii. 100 μL of the Enzyme Conjugate was pipetted into all wells.
iii. Incubated for 30 min at RT (20-25°C) on a shaker (approx. 600 rpm).
iv. The contents of well were discarded or aspirated, and each well was washed 4 times thoroughly with 300 μL Wash Buffer. Blotted dry by tapping the inverted plate on absorbent material.
v. 100 μL of the Substrate were pipetted into all wells and incubate for 20-30 min at RT (20-25°C) on a shaker (approx. 600 rpm). Avoid exposure to direct sunlight.
vi. 100 μL of the Stop Solution were pitted to each well and shake the microtiter plate to ensure a homogeneous distribution of the solution. The absorbance of the solution in the wells was read within 10 minutes, using a microplate reader set to 450 nm with a reference wavelength between 620 nm and 650 nm.
Statistical Analysis
Analysis of Variance (ANOVA) test was applied for statistical evaluation of the obtained results of each detected residue in the examined samples of salted and smoked fish products according to Feldman et al., (2003).
Results
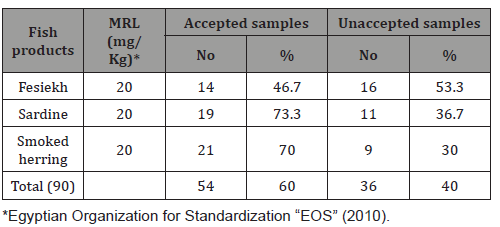
It is evident from the results recorded in Table 1 that the Histamine values in examined fish samples were varied from2.9- 36.1mg/kg with an average of 20.76 ± 0.54 for Fesiekh;2.2-29.8mg/ kg with an average of 15.49 ± 0.31for sardine and1.4-21.7mg/kg with an average of 9.82 ± 0.26 for smoked herring. The differences between the examined samples of different fish species were high significant (P<0.01). Table 2 revealed that46.7%.73.3% and 70%of the examined samples of Fesiekh, sardine and smoked herring were accepted, however, 53.3%, 36.7% and 30% of such samples were unaccepted, respectively.
Table 1: Concentrations of histamine (mg/Kg) in the examined samples of salted and smoked fish (n=30).
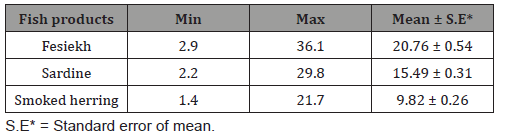
Table 2: Validity of the examined salted and smoked fish based on their histamine contents (n=30).
Discussion
The results recorded that the histamine mean values in examined fish samples were20.76 ± 0.54, 15.49 ± 0.31 and 9.82 ± 0.26mg/kg, unaccepted samples were53.3%, 36.7% and 30%for fesiekh, sardine and smoked herring, respectively.
Results of histamine in fesiekh nearly like those obtained by Edris 2014 [10] (18.06 ± 0.99 mg/kg) who investigated histamine in 90 samples of fesiekh, sardine and melloha (30 of each) collected from different retail markets.
Lower than that recorded by Nader [11] (33.21±1.15 mg/100g) who investigated histamine in 20 samples of feisiekh were collected from markets in kafr El- Sheikh. Higher than that recorded by Elshafey-Wesam [12] (16.80± 0.31 mg/kg). measured histamine in 15 samples of Mugil Cephalus.
Results of histamine in sardine lower than [13](29.65±1.41 ppm) estimated the level of histamine in sardine samples using HPLC,[10] (23.51±1.21 mg/kg)who investigated histamine in 30 samples of sardine collected from different retail markets,[11] (28.14±1.02mg/100g)[12] (28.74 ± 0.52 mg/kg).
Results of histamine in smoked herring lower than [11] (23.12±0.86 mg /100g).Fish commonly implicated in histamine fish poisoning include both scombroid (mackerel, tuna and saury) and non- scombroid (sardine, anchovies, blue fish, as they contain large amount of free histadine [14].sardines characterized by the presence of high levels of free histamine in their muscle, also according to the season of the year, genetics, environment, food, sex, physiological stage, storage period and sampled tissue [15]. High levels of biogenic amines can be prevented by the application of good hygiene practices and proper temperature during handling, delivery and storage [16].Biogenic amines can be used as quality index, once they are formed by bacterial activity and are resistant to thermal treatment, thus reflecting the quality of the raw material and hygienic conditions of food processing [17] and [18].Acceptability of examined fish samples based on their levels of histamine according to EOS (2010) the results revealed that46.7%.73.3% and 70%of the examined samples of fesiekh, sardine and smoked herring were accepted, however, 53.3%, 36.7% and 30% of such samples were unaccepted, respectively. Unfortunately, unhygienic practices, insufficient refrigeration cause increase susceptible to contamination by BAs-producing microorganisms and other spoilage bacteria (proteolytic and lipolytic) leading to rapid spoil agenda outbreaks of fish poisoning [19].
Conclusion
As conclusion, histamine is the main marker for the evaluation of quality and safety of fish. Also, the application of good hygiene practices and proper temperatures during handling, delivery and storage reduce the bacterial growth and multiplication with further undesirable changes [20].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- El-Moselhy KM (2000) Accumulation of copper, cadmium and lead in some fish from the Guif of suez. Egyptian Journal of Aquatic Biology and Fisheries 3(1).
- Tombelli S, Mascini,M (1998) Electrochemical biosensors for biogenic amines: a comparison between different approaches. Analytica Chimica Acta Journal 385(3): 277-284.
- Patange SB, Mukundan MK, Ashok Kumar K (2005) A simple and rapid method for colorimetric determination of histamine in fish flesh. Food Control Journal16: 465-472.
- Surya T, Sivaraman B, Alamelu VA, Priya tharshini A, Arisekar U, et al. (2019) Rapid Methods for Histamine Detection in Fishery Products. International Journal of Current Microbiology and Applied Sciences 8(3): 2035-2046.
- FDA (2011) Fish and fishery products, hazards and controls guide. (3rd edn), pp. 73-90.
- Ferran M, Yébenes M (2006) Flushing associated with scombroid fish poisoning. Dermatol Online Journal 12(6): 15.
- Hungerford JM(2010) Scombroid poisoning: a review. Toxicon Journal 56(2): 231-243.
- Feng C, Teuber S, Gershwin ME (2016) Histamine (Scombroid) Fish Poisoning. Clinical reviews in allergy & immunology Journal. 50(1): 64-69.
- Harmelin Y, Hubiche T, Pharaon M, Del Giudice P (2018) [Three cases of scombroid poisoning].Annales de Dermatologie et de Venereologie Journal 145(1): 29-32.
- Edris AA, Amin Reham A, Naseif Marionette Z, AbdelFatah- Ebtsam M (2014) Evaluation of Retiled Salted Fish according to Egyptian Standard.Benha Veterinary Medical Journal. 27: 168‐176.
- Nader YM, ElBahy-Engy F, Anter-Dalia M (2016) Biogenic Amines in Salted and Smoked Herring Fish. Global Veterinaria Journal. 16 (6): 525-529.
- Elshafey Wesam S, Ibrahim Hemmat M, Hassan MA, Maarouf AA (2018) Assessment of Histamine and Putrescine Residues in Fish and Shellfish. Benha Veterinary Medical Journal 34(3): 178-187.
- Walaa (2016) Biogenic Amines as quality indices of imported frozen fish.
- Lehane L, Olley J (2000) Histamine fish poisoning revisited. International Journal of Food Microbiology 58(1-2): 1-37.
- Lee Y, Kung H, Lin C, Hwang C, Tsai Y (2012) Histamine production by Enterobacter aerogenes in tuna dumpling stuffing at various storage Food Chemistry Journal 131: 405-412.
- Visciano P, Schirona M, Tofalo R, Suzzi G (2012) Biogenic amine in raw and processed sea food. Department of Food science, University of Teramo, Italy. Frontiers in Microbiology 10: 33-89.
- Shaltout Fa Hashim M M F (2002) Histamine in Cannei, Smoked and Salted Fish Products. Benhavet. Med 13(I): 1-10.
- Sagratini G, Fernández-Frazón M, Berardinis F, Font G, Vittori S, Mañes J (2012) Determination of eight un derivatized biogenic amines in fish by solid phaseextraction and liquid chromatography tandem mass spectrometry. Food Chemistry Journal 132: 537-543.
- Önal A (2006) Current analytical methods for determination of biogenic amines in foods. Food Chemistry Journal 103(4): 1475-1786.
- Zhai H, Yang X, Li L, Xia G, Cen J, et al. (2012) Biogenic amines in commercial fish and fish products sold in southern china. Food Control Journal 25: 303-308.
-
Hassan M, Shaltout FA, Saqur N. Histamine in Some Fish Products. Arch Animal Husb & Dairy Sci. 2(1): 2020. AAHDS. MS.ID.000527.
-
ELISA, Histamine compounds, Salted, Smoked fish.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






