 Research Article
Research Article
Role of Transcarotid Artery Revascularization (TCAR) as a Carotid Revascularization Option
FIA Danish*, Saeeda Yasmin, Ahmad Ehsan Rabani, Fazal-e-Rabi Subhani, Salman Shafi Koul, Naila Tabassum and Muhammad Amer
Yeovil District Hospital, Higher Kingston, UK
FIA Danish, Yeovil District Hospital, Higher Kingston, Yeovil Somerset, UK.
Received Date: February 09, 2023; Published Date: March 02, 2023
Transcarotid artery revascularization (TCAR) is a hybrid carotid revascularization procedure in which carotid stenting is accomplished by making an incision at the base of the neck & surgically exposing the common carotid artery. The evidence base for TCAR safety & efficacy comes from PROOF, ROADSTER, & ROADSTER 2 studies. The Society for Vascular Surgery guidelines for the treatment of carotid artery disease (2022) opine TCAR to be at least equivalent to Carotid Endarterectomy (CEA) in terms of safety & efficacy, with some potential improvements. In comparison with Transfemoral Carotid Artery Stenting (TF-CAS), the overall data demonstrates better outcomes with TCAR. In high-risk subjects like elderly more symptomatic patients with higher concurrent medical comorbidities, TCAR in fact appears to be preferable to both CEA & TF-CAS. Whereas this technique appears safe in the short-term, long-term vessel patency requires prospective follow-up studies.
Keywords:Extracranial carotid atherosclerotic disease; stenosis of supra-aortic arch vessels; carotid revascularization options; transcarotid artery revascularization
Introduction
Extracranial carotid atherosclerotic disease is treated with maximal medical therapy & carotid revascularization, if indicated. The conventional carotid revascularization options include Carotid Endarterectomy (CEA) and Carotid Artery Stenting (CAS). Whereas CAS most often involves percutaneous carotid stenting via the transfemoral approach (TF-CAS), literature also describes the infrequently-chosen transcervical approach (TC-CAS). Transcarotid artery revascularization (TCAR) on the other hand is a distinct ‘hybrid’ procedure in which carotid stenting is accomplished by making an incision at the base of the neck & surgically exposing the common carotid artery (CCA) [1,2]. In patients with extracranial carotid atherosclerotic disease, the general indications for carotid revascularization remain the same regardless of the revascularization approach (CEA, TF-CAS, or TCAR). In this article, the advantages & disadvantages, technical aspects, and clinical outcomes of TCAR are discussed.
Methods
A comprehensive search of PubMed & EMBASE from Jan 2000 to January 2023 was made using 4 search items: extracranial carotid atherosclerotic disease, stenosis of supra-aortic arch vessels, carotid revascularization options, & transcarotid artery revascularization. The search items were combined using the Boolean operator.
Discussion
Carotid revascularization using transcarotid Stenting System
TCAR stenting system includes an arterial sheath, a venous sheath, and a flow controller with a built-in filter.3 After surgical exposure of CCA, the arterial sheath is directly inserted into the CCA. The arterial sheath is connected to the flow controller, which in turn is connected to the venous sheath placed percutaneously into either the right or left Common Femoral Vein (CFV). Next CCA is surgically occluded proximal to the arterial sheath inducing blood flow reversal away from the brain. Owing to differential pressures within the arterial & venous systems, the blood starts flowing through the TCAR stenting system & returns the body via the venous sheath. The main advantage of this dynamic-flow-reversal system is prevention of iatrogenic distal embolization & stroke.
Although stents typically used for TF-CAS can also be employed during TCAR (provided they fit the arterial sheath), ENROUTE stent is the only stent specifically approved for TCAR in the United States (US). Compared with other stent platforms used for CAS, ENROUTE stent has a shorter delivery system (57 cm) & has the smallest cell size amongst the currently available open-cell design stents [2,4,5].
Anatomic requirements for TCAR
TCAR can only be performed safely if following anatomic requirements are all met on ultrasonography:
1. Distance between supraclavicular surgical access site & the targeted vascular lesion is >5 cm
2. CCA diameter is >6 mm
3. CCA puncture site for arterial sheath & the proximal occlusion site are both free of any significant disease like thrombus or calcification (within 1 cm of either site).
Whereas Computed Tomography Angiography (CTA) can be used to ascertain the anatomic requirements for TCAR, it may underestimate the distance available between the access site to the target lesion. In one retrospective review of 118 patients who underwent CEA or CAS, 72% of carotid arteries retrospectively fulfilled the anatomic requirements criteria (listed above) on CTA and thus were opined eligible for TCAR [6]. Notably in this analysis, of the arteries deemed high risk for TF-CAS, 69% were found eligible for TCAR. In another similar study involving 433 patients, 85% of carotid arteries fulfilled the anatomic requirements criteria on CTA and thus deemed eligible for TCAR [7]. Both these studies concluded that in significant majority of patients selected for carotid revascularization (via CEA or CAS), the anatomic requirements for TCAR are simultaneously met, making TCAR a viable alternative therapeutic option.
Evidence-base for TCAR safety & efficacy
The evidence base for TCAR safety & efficacy comes from PROOF, ROADSTER, & ROADSTER 2 studies. Initial evidence of TCAR safety & efficacy came from The Silk Road Medical Embolic PROtectiOn System: First-In-Man (PROOF) study in which all enrolled patients successfully underwent TCAR and developed no major strokes, myocardial infarctions (Mis), or deaths during the 30-day follow-up period [8]. Of the 31 patients who underwent postprocedural MRI (DWI) examination, 5 patients (16%) demonstrated radiologic evidence of new ischemic brain lesions but no clinical sequelae. Reverse Flow Used During Carotid Artery Stenting Procedure (ROADSTER) study enrolled 208 patients who either had symptomatic ≥50 percent stenosis or asymptomatic ≥70 percent stenosis and were considered high-risk for complications from CEA [9]. This prospective, single-arm, multicentre clinical trial was performed to evaluate the safety & efficacy of ENROUTE Transcarotid neuroprotection system (NPS) (Silk Road Medical Inc, Sunnyvale, Calif). Results demonstrated initial acute device & technical success rate of 99% (140 of 141). By hierarchical analysis, the all-stroke rate in the pivotal group was 1.4% (2 of 141), composite rate of stroke/death was 2.8% (4 of 141), and composite rate of stroke/death/MI was 3.5% (5 of 141). ROADSTER 2 was also a prospective, single-arm, multicentre clinical trial that enrolled 692 patients who either had symptomatic ≥50 percent stenosis or asymptomatic ≥80 percent stenosis and were considered highrisk for complications from CEA [10]. The results demonstrated successful achievement of primary endpoints (i.e. technical success plus absence of stroke/death/MI) within the 30-day postoperative period in 97.9% subjects. The 30-day perioperative stroke rate was 0.6%, the composite rate of stroke/death was 0.8%, and composite rate of stroke/death/MI was 1.7%. The trial thus demonstrated high technical success by a majority of operators new to TCAR technology at the start of the trial, excellent early outcomes, & low rates of post-procedure stroke & death.
TCAR Surveillance Project (TSP) & comparison of TCAR with other carotid revascularization options
TCAR Surveillance Project (TSP) was created in 2016 as an open-ended registry to provide & compare real-world patient outcomes for TCAR versus alternative carotid revascularization procedures. Originally TSP only enrolled ‘high-risk patients (either asymptomatic ≥80% stenosis or symptomatic ≥50% stenosis). Since June 2022 however, ‘standard risk’ patients (either asymptomatic ≥70% stenosis or symptomatic ≥50% stenosis) are enrolled in this registry. All TSP enrolled patients have their clinical data entered in the Society for Vascular Surgery - Vascular Quality Initiative (SVS-VQI). Based on the available evidence, the Society for Vascular Surgery guidelines for the treatment of carotid artery disease (2022) opine TCAR to be at least equivalent to CEA in terms of safety & efficacy, with some potential improvements [11]. In comparison with TF-CAS, the overall data demonstrates better outcomes with TCAR [11]. In high-risk patients (advanced age; more symptomatic disease; higher incidence of concurrent medical/ cardiac comorbidities), TCAR in fact appears to be preferable to both CEA & TF-CAS [11].
TCAR vs CEA: In one TCAR Surveillance Project (TSP) study comparing in-hospital TCAR & CEA outcomes, the unadjusted analysis of the two therapeutic interventions revealed no difference in the rate of stroke (1.4% vs 1.2%), in-hospital death (0.3% vs 0.3%), 30-day death (0.9% vs 0.4%), or MI (1.1% vs 0.6%) [12]. On adjusted analysis, no difference in terms of stroke/death or stroke/ death/MI was observed either. Another TSP study yielding similar results demonstrated patients in TCAR cohort to be older (median age 74 vs 71 years; P < .001), more symptomatic (32% vs 27%; P < .001), and suffering from more medical comorbidities including coronary artery disease (55% vs 28%; P < .001), chronic heart failure (20% vs 11%; P < .001), chronic obstructive pulmonary disease (29% vs 23%; P < .001), and chronic kidney disease (39% vs 34%; P = .001). TCAR operative times were found to be >30 minutes shorter than traditional CEA and associated with lower incidence of cranial nerve injuries (0.6% vs 1.8%; P < .001) & shorter postop hospital stays (>1 day post-op hospital stay = 27% vs 30%; P .046). Since, compared with CEA, TCAR is more often performed under local or regional anaesthesia, the theoretical cognitive & cardiovascular risks associated with general anaesthesia are presumably minimized in TCAR. On the flip side, given higher rates of prior ipsilateral CEA, the probability of redo carotid intervention appears higher in the TCAR patients (16% vs 1.9%; P < .001) [13]. In conclusion, these comparative-effectiveness studies in patients who underwent TCAR or CEA opined TCAR to be a safe alternative to CEA in certain patients with carotid artery stenosis.
TCAR vs TF-CAS: Given that both TF-CAS & TCAR involve carotid stenting, the carotid vascular characteristics deemed prohibitive for TF-CAS are also often considered absolute or relative contraindications for TCAR. Such characteristics may include severe carotid tortuosity, small internal carotid artery (that may not accommodate commercially-available arterial stents), severe plaque calcification, circumferential carotid plaque, visible thrombus within the lesion (detected on preoperative imaging), near occlusion of the carotid artery & active infection. Having said this, since TCAR involves accessing CCA at the base of the neck, unlike TF-CAS, abnormalities in aortic anatomy (e.g. severe aortic arch calcification or arch tortuosity, especially type III arches) is almost irrelevant in TCAR. Given that the incision in TCAR is small & often well away from the worst radiation areas, in many patients with history of prior neck radiation or surgery, TCAR can be conducted safely. Given likely significant oropharyngeal bacterial colonization of neck skin, tracheostomy is considered a relative contraindication for TCAR.
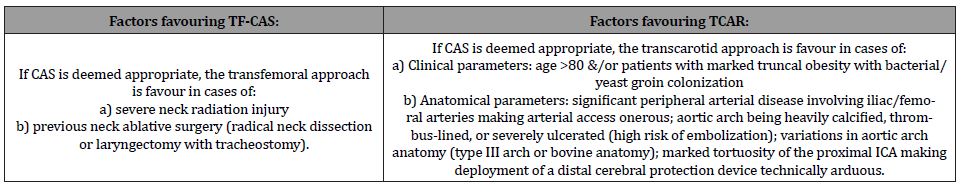
In one TSP study that compared TF-CAS vs TCAR in-hospital outcomes, TCAR was found associated with lower rates of TIA/ stroke (1.9% vs 3.3%) & TIA/stroke/death (2.2% vs 3.8%) [14]. These results were yielded despite TCAR patients being significantly older, more symptomatic, and suffering from higher cardiac comorbidities. The prospective incidence of recurrent stenosis was also found to be lower in TCAR patients. On the flip side, compared with TF-CAS, TCAR procedure was more likely to be performed under general anaesthesia (79% vs 12%). In a later TSP study comparing 5251 TCAR procedures with 6640 TF-CAS procedures, TCAR was associated with a lower risk of in-hospital stroke or death (1.6% vs 3.1%), stroke (1.3% vs 2.4%), and death (0.4% vs 1%) [15]. Concerning perioperative MI risk, no statistically significant difference was found (0.2% vs 0.3%). Using Kaplan- Meier life-table estimation, TCAR was found to be associated with lower risk of ipsilateral stroke or death at one year (5.1% vs 9.6%). Please see Table 1 for specific factors that would either favour TFCAS or TCAR.
Table 1:Choosing the right approach in CAS-appropriate patients: TF-CAS vs TCAR.
TCAR for supra-aortic lesions
Endovascular treatment has mostly replaced open reconstruction of proximal brachiocephalic & left common carotid ostial arterial stenoses. In a multicentre case series of 16 patients (11 symptomatic patients, 11 patients with single lesions of the supra-aortic arch vessels, & 5 patients with tandem stenotic lesions), ENROUTE Transcarotid NPS was used for endovascular stenting of a total of 21 lesions (7 innominate artery, 1 right CCA, 8 left CCA, & 5 ICA) [16]. For proximal lesions, the arterial sheath was directed in a retrograde fashion, with CCA clamping performed distal to the insertion site. For tandem lesions, the arterial sheath was next inserted in an antegrade fashion through a separate CCA puncture as per standard TCAR. Results demonstrated technical success in all cases, and no TIA/strokes during the post-procedure 30 days. The data thus supported technical feasibility of ENROUTE Transcarotid NPS for endovascular stenting of single & tandem stenotic lesions of the supra-aortic arch vessels.
Patient preparation for TCAR
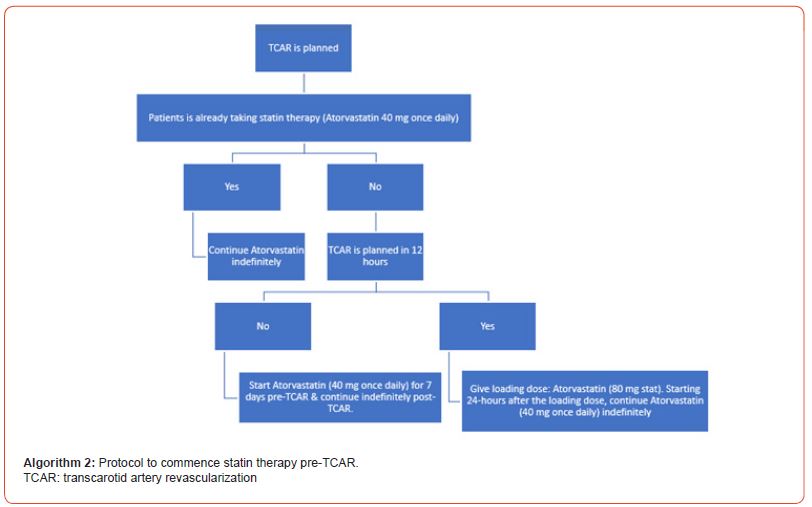
Pre-TCAR drug therapy: dual antiplatelet therapy and statins: Initiation of dual antiplatelet therapy (DAPT) & statin therapy is recommended in all patients before undergoing TCAR – see Algorithms 1 & 2.

Pre-treatment: Since hypotension & bradycardia may occur during TCAR owing to stimulation of baroreceptors, it is generally recommended that all TCAR patients are pre-treated with 0.2 mg of glycopyrrolate (glycopyrronium). Since the recommended systolic blood pressure is 140-160 mmHg, depending upon the initial response, an additional dose of 0.2 mg of glycopyrrolate (or top up with Atropine even) may be needed. Being antimuscarinic anticholinergic agents, both these drugs induce a dose-related increase in heart rate & blood pressure (systolic, diastolic, & mean). Although glycopyrrolate is approximately twice as potent as atropine in increasing heart rate, the peak effect is achieved quicker with Atropine (2-6 minutes vs 3-7 minutes).
Technique: TCAR requires making a short (3 cm) transverse or longitudinal supraclavicular incision centered between the sternal & clavicular heads of the sternocleidomastoid muscle. Carotid artery access at this point obviates the need to pass catheters through an anatomically challenging or diseased aortic arch. After making the incision, carotid sheath is reached by vigilant dissection through the avascular plane between the sternal & clavicular heads of the sternocleidomastoid muscle. Once reached, either a vascular loop or umbilical tape can be placed around CCA to aid vascular clamping while simultaneously making sure to avoid injuring the Vagus nerve. Next (& before arterial sheath insertion), immediate anticoagulation is achieved with intravenous (IV) heparin (target activated clotting time [ACT] >250 seconds); in case of heparin allergy, alternative IV agents e.g. argatroban or bivalirudin can be used. Next, a 5-0 polypropylene ‘U’ or ‘Z’ suture is placed on CCA at the intended puncture site (this would aid in later puncture site closure & haemostasis). Using a micropuncture access kit (containing appropriate length wires & a sheath), the CCA is then accessed & initial angiography performed to delineate the anatomy. Next the arterial sheath is inserted in CCA & venous sheath in either right or left common femoral vein. The two sheaths are then connected using the intervening flow controller device. Additional angiography is then obtained to further delineate the target lesion. CCA is next occluded proximal to the arterial sheath location either by using a vascular clamp or tightening a vessel loop. This allows reversal of blood flow away from ICA into the venous system. Flow reversal is confirmed by infusing saline into the venous sheath & seeing if blood flow through the neuroprotection system clears the saline. Next the ICA stenosis is crossed with a 0.014 inch diameter wire. Before deploying the stent, the target ICA lesion is predilated to the nominal ICA diameter to ensure smooth luminal gain. The stent is then deployed across the target lesion. Postdilation (after stent deployment) is generally dissuaded unless there is significant residual stenosis of >30%. Next comes a mandatory wait of at least two minutes to allow clearance of any debris potentially resulting from manipulation of the lesion. Finally, a completion arteriography is performed in at least two orthogonal views to confirm adequate stent lumen expansion & apposition to the arterial wall. The procedure is completed, by removing the wire, restoring antegrade flow in CCA, disconnecting flow controller, removing arterial sheath (& obtaining haemostasis by tying down the previously placed suture), removing venous sheath (& obtaining haemostasis by manual pressure), and closing the neck incision in layers in the standard fashion.
Post-treatment: After completing the procedure, it is generally recommended to reverse anticoagulation using protamine sulfate. Such reversal is known to significantly lower the bleeding complications risk (2.8% vs 8.3%), lower rate of need for postprocedural blood transfusions (1.1% vs 2%) & lower risk of need for surgical intervention to establish haemostasis (1% vs 3.6%) [17]. Encouragingly, protamine use is not known to increase the risk of thrombotic events (TIA/stroke/MI), heart failure exacerbation or postoperative hypotensive hemodynamic instability [17].
Post-TCAR care: Post-TCAR care involves transferring the patient to a monitored setting to allow frequent blood pressure monitoring & neurologic assessment. Compared with CEA, carotid artery stenting is known to be associated with a higher rate of postprocedural hypotension [18, 19]. This is likely owing to continued stretching of the baroreceptors in the carotid bulb by the implanted stent. Whereas in one study, clinically significant postprocedural hypotension was not associated with increased risk of postprocedural strokes (0.8% vs 0.6%, P = .75) or recurrent neurologic symptoms (0.4% vs 0.3%, P = .55), it was correlated with increased incidence of postprocedural MI (2.1% vs 0.5%, P = .022), higher mortality (2.1% vs 0.1%, P < .001), and >2 days length of stay (46.3% vs 27.4%, P = .01) [19]. To avoid these sequelae, close monitoring & aggressive treatment of post-procedural hypotension is an absolute essential (target systolic BP 100-150 mmHg). On the flip side, postprocedural hypertension should also be avoided lest neck hematoma should develop. DAPT & statin therapy is continued post-TCAR (Algorithms 1 & 2).
Post-TCAR follow-up
Similar to TF-CAS, routine duplex imaging is performed post- TCAR to identify restenosis. The first scan within three months post-TCAR is intended to establish a new baseline for future comparison. Whereas more frequent scans are warranted if a contralateral stenosis is present, generally speaking follow-up scans are performed every six months for two years and thereafter annually till a confirmation of absence of restenosis is achieved in two consecutive annual scans [20].
TCAR complications:
Complications associated with carotid revascularization can be grouped into:
1. Complications precipitated by carotid revascularization: These primarily include TIAs & strokes (both ischaemic & haemorrhagic).
2. Complications related to patient comorbidities: These include systemic complications like MI & worsening chronic kidney disease.
3. Complications related to the stent: These include in-stent restenosis & stent fracture.
4. Complications specific to the revascularization approach. These include complications related to the arterial access or related to the embolic protection system.
Stroke: Stroke represents the most serious acute complication post-TCAR. The underlying mechanisms alone or in combination may include thromboembolism, hypoperfusion syndrome, hyperperfusion syndrome, stent thrombosis, non-compliance with antiplatelet therapy, and intracerebral haemorrhage.
Multiple strategies are employed in both TF-CAS & TCAR cases to mitigate iatrogenic stroke risk. These includes appropriate patient selection, perioperative treatment with DAPT & statin therapy, intraoperative IV Heparin, & strict postprocedural BP control. Despite this, compared with CEA & TF-CAS, post-TCAR stroke rates appear to be lower; in one study, stroke rates were 1.3% & 2.4% in the TCAR & TF-CAS groups respectively [15]. A possible explanation for this difference is the obviation of the need to traverse aortic arch during TCAR. Aortic arch being home to considerable concurrent atherosclerotic thromboembolic debris is deemed the most likely incriminated site to confer embolization in TF-CAS subjects. Flow reversal established during TCAR that permits any debris to flow away from brain probably also contributes a protective effect.
Cerebral hyperperfusion syndrome: In cases of atherosclerotic carotid stenosis, chronic maximal dilatation of collateral vasculature helps maintain (almost) sufficient cerebral blood flow. After carotid revascularization when cerebral blood flow is suddenly restored to a normal (or high) perfusion pressure within the previously hypoperfused hemisphere, the previously dilated collateral vasculature may fail to vasoconstrict immediately & sufficiently. This in turn may induce cerebral oedema & (in the worst case scenario) ICH. Although an uncommon after-effect of CEA (incidence ranging from <1% to as high as 3% in various reports), hyperperfusion is probably the most likely underlying cause of intracerebral haemorrhages & seizures in the first two weeks post-CEA [21-24].
Antecedent hypertension & suboptimal perioperative BP control: seem to increase the risk of hyperperfusion syndrome. Extrapolating data from CEA cohorts, the risk of hyperperfusion syndrome is further increased if revascularization is performed in 80% carotid stenosis cases.
Other complications: may include bleeding, hematoma, arterial dissection, & nerve injury. Complications not specific to TCAR include MI, renal failure (precipitated by IV contrast), stent fractur, & carotid thrombosis/restenosis.
Learning curve for TCAR:
Just like any other procedure, whereas increasing experience does improve operative efficiency, vascular surgeons who are already well experienced in CEA & TF-CAS are known to achieve low complication rates (e.g. stroke, MI, death) early in their experience with TCAR even when TCAR is performed under GA. In one study involving 188 consecutive patients treated with TCAR, operating surgeons managed to decrease the procedural time from 79 to 71 minutes & duration of flow reversal from 13 to 9 minutes after 15 cases suggesting a relatively short learning curve [25]. The incidence of operative complications remained similarly low with both early & late experience. Based on the data from TSP, clinician’s operative experience & efficacy was divided into four levels: novice (1 to 5 cases), intermediate (6 to 20 cases), advanced (20 to 30 cases), and expert (>30 cases) [26]. An international quality assurance database of 18,240 procedures performed by 1273 physicians highlighted two ‘proficiency parameters i.e. (a) flow reversal target time of <13.1 minutes and (b) skin-to-skin time of 81 minutes [27]. These proficiency parameters considered associated with lower adverse event rates were generally met after operative experience of ≥26 cases.
Conclusion
Retrograde TCAR in patients with a short CCA (<5 cm from clavicle to carotid bifurcation) or plaque or excessive angulation of the CCA is not recommended. In carefully selected patients, however, the retrograde approach seems promising for endovascular stenting of single & tandem stenotic lesions of supra-aortic arch vessels. Whereas the technique appears safe in the short-term, long-term vessel patency would require prospective follow-up of these patients.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Kwolek CJ, Jaff MR, Leal JI (2015) Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg 62(5): 1227-1234.
- Pinter L, Ribo M, Loh C (2011) Safety and feasibility of a novel transcervical access neuroprotection system for carotid artery stenting in the PROOF Study. J Vasc Surg 54(5): 1317-1323.
- Malas MB, Leal J, Kashyap V, Cambria RP, Kwolek CJ, et al. (2017) Technical aspects of transcarotid artery revascularization using the ENROUTE transcarotid neuroprotection and stent system. J Vasc Surg 65(3): 916-920.
- Leal I, Orgaz A, Flores Á (2012) A diffusion-weighted magnetic resonance imaging-based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg 56(6): 1585-1590.
- Alvarez B, Matas M, Ribo M, Maeso J, Yugueros X, et al. (2012) Transcervical carotid stenting with flow reversal is a safe technique for high-risk patients older than 70 years. J Vasc Surg 55(4): 978-984.
- Wu WW, Liang P, O’Donnell TFX (2019) Anatomic eligibility for transcarotid artery revascularization and transfemoral carotid artery stenting. J Vasc Surg 69(5): 1452-1460.
- Kumins NH, King AH, Ambani RN (2020) Anatomic criteria in the selection of treatment modality for atherosclerotic carotid artery disease. J Vasc Surg 72(4): 1395-1404.
- Kashyap VS, Schneider PA, Foteh M (2020) Early Outcomes in the ROADSTER 2 Study of Transcarotid Artery Revascularization in Patients With Significant Carotid Artery Disease. Stroke 51(9): 2620-2629.
- AbuRahma AF, Avgerinos ED, Chang RW (2022) Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J Vasc Surg 75(1S): 4S-22S.
- Columbo JA, Martinez-Camblor P, O’Malley AJ (2021) Association of Adoption of Transcarotid Artery Revascularization With Center-Level Perioperative Outcomes. JAMA Netw Open 4(2): e2037885.
- Schermerhorn ML, Liang P, Dakour-Aridi H (2020) In-hospital outcomes of transcarotid artery revascularization and carotid endarterectomy in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 71(1): 87-95.
- Malas MB, Dakour-Aridi H, Wang GJ (2019) Transcarotid artery revascularization versus transfemoral carotid artery stenting in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 69(1): 92-103.e2.
- Schermerhorn ML, Liang P, Eldrup-Jorgensen J (2019) Association of Transcarotid Artery Revascularization vs Transfemoral Carotid Artery Stenting With Stroke or Death Among Patients With Carotid Artery Stenosis. JAMA 322(23): 2313-2322.
- Balceniuk MD, Hosn MA, Corn RS (2020) Endovascular stenting of supra-aortic lesions using a transcarotid retrograde approach and flow reversal: A multicenter case series. J Vasc Surg 71(6): 2012-2020.e18.
- Liang P, Motaganahalli RL, Malas MB (2020) Protamine use in transcarotid artery revascularization is associated with lower risk of bleeding complications without higher risk of thromboembolic events. J Vasc Surg 72(6): 2079-2087.
- Lin PH, Zhou W, Kougias P, El Sayed HF, Barshes NR, et al. (2007) Factors associated with hypotension and bradycardia after carotid angioplasty and stenting. J Vasc Surg 46(5): 846-853.
- Park BD, Divinagracia T, Madej O (2009) Predictors of clinically significant postprocedural hypotension after carotid endarterectomy and carotid angioplasty with stenting. J Vasc Surg 50(3): 526-533.
- Zierler RE, Jordan WD, Lal BK (2018) The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J Vasc Surg 68(1): 256-284.
- Coutts SB, Hill MD, Hu WY (2003) Hyperperfusion syndrome: toward a stricter definition. Neurosurgery 53(5): 1053-1058.
- Youkey JR, Clagett GP, Jaffin JH, Parisi JE, Rich NM (1984) Focal motor seizures complicating carotid endarterectomy. Arch Surg 119(9): 1080-1084.
- Naylor AR, Ruckley CV (1995) The post-carotid endarterectomy hyperperfusion syndrome. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg 9(4): 365-367.
- Reigel MM, Hollier LH, Sundt TM, Piepgras DG, Sharbrough FW, et al. (1987) Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg 5(4): 628-634.
- King AH, Kumins NH, Foteh MI, Jim J, Apple JM, et al. (2019) The learning curve of transcarotid artery revascularization. J Vasc Surg 70(2): 516-521.
- Kashyap VS, King AH, Liang P (2020) Learning Curve for Surgeons Adopting Transcarotid Artery Revascularization Based on the Vascular Quality Initiative-Transcarotid Artery Revascularization Surveillance Project. J Am Coll Surg 230(1): 113-120.
- Lal BK, Mayorga-Carlin M, Kashyap V (2022) Learning curve and proficiency metrics for transcarotid artery revascularization. J Vasc Surg 75(6): 1966-1976.e1.
-
FIA Danish*, Saeeda Yasmin, Ahmad Ehsan Rabani, Fazal-e-Rabi Subhani, Salman Shafi Koul, Naila Tabassum and Muhammad Amer. Role of Transcarotid Artery Revascularization (TCAR) as a Carotid Revascularization Option. On J Cardio Res & Rep. 7(2): 2023. OJCRR.MS.ID.000656.
-
Extracranial carotid atherosclerotic disease, stenosis of supra-aortic arch vessels, carotid revascularization options, transcarotid artery revascularization, Carotid artery, Major strokes, Myocardial infarctions, Cardiac comorbidities, Anaesthesia, Cardiovascular risks, Heart rate Blood pressure
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






