 Case Report
Case Report
Recurrent Transient Ischemic Attack in A Young Lady with Embolic Stroke of Undetermined Source and Patent Foramen Ovale: Quo Vadis- Direct Cause, Risk Factor, or Incidental Finding?
Richmond R Gomes*
Associate Professor, Medicine, Ad-din Women’s Medical College Hospital, Bangladesh
Richmond R Gomes, Associate Professor, Medicine, Addin Women’s Medical College Hospital, Dhaka, Bangladesh.
Received Date: April 10, 2023; Published Date: May 02, 2023
Approximately 40% of ischemic strokes have no clearly definable etiology and are termed cryptogenic strokes. Patent foramen ovale (PFO), a small communication between the left and right atria, is a common benign finding in healthy subjects. Its prevalence is higher in patients with cryptogenic stroke. There is conflicting data and it remains uncertain whether PFO is the direct cause, a risk factor or an incidental finding. Potential stroke mechanisms include paradoxical embolism from a venous clot which traverses the PFO, in situ clot formation within the PFO, and atrial arrhythmias due to electrical signaling disruption. Main risk factors linked with PFO-attributable strokes are young age, PFO size, right-to-left shunt degree, PFO morphology, presence of atrial septal aneurysm, intrinsic coagulation-anticoagulation systems imbalance, and co-existence of other atrial abnormalities, such as right atrial septal pouch, Eustachian valve and Chiari’s network. These may act independently or synergistically, multiplying the risk of embolic events. Recent trials have shown that closure of PFO, especially if associated with an atrial septal aneurysm and/or a large interatrial shunt, may reduce the risk of recurrent stroke as compared to medical treatment. PFO presence in young patients with cryptogenic stroke should be considered as etiologically suspect. In this study, we report the case of a 32-year-old woman with diagnosis of recurrent transient ischemic attack (TIA) due to PFO. The aim of this report is to discuss the relevant aspects of the PFO and the cryptogenic stroke, its clinical presentation, diagnosis, management and recurrence.
Keywords:Cryptogenic stroke; Paradoxical embolism; Transient ischemic attack; Atrial arrythmia
Introduction
There are different possible causes of stroke, but approximately 20% are of cardioembolic origin [1]. If it is not discovered what caused the ischemic event, it is classified as cryptogenic. Strokes of unknown cause are particularly common in young adults, accounting for 10-40% depending on the population [2]. The atrial septum is formed during the embryogenesis by two membranes growing from the atrial walls (septum primum- a flap that forms the floor of the fossa ovalis and septum secundum-an infolding of the atrial wall on the right atrialside), leaving an oval shaped fenestration (foramen ovale).This communication allows blood from the inferior caval vein – directed preferentially to the foramen ovale by the Eustachian valve – to bypass the pulmonary circulation into the left heart (Figure 1). Following decreased pulmonary resistance and increased left atrial (LA) pressure at birth, the septum primum adheres to the fossa ovalis rim, and obliteration of the foramen ovale is usually complete by the first year of life 3]. The foramen ovale is sealed during the first year of life by the fusion of the two membranes. The failure of this process leads to an interatrial slit-like channel, the patent foramen ovale (PFO) [4,5,6] (Figure 1). PFO is considered to be a subclass of ostium secundum defects [7]. PFO is present in 25%-35% of the general population, tending to decline with increasing age, andis the most frequent cause of R-L shunt in adults [5,8,9,10]. Although most of the times PFO is “innocent,” it has been associated with cryptogenic stroke (CS), migraine, peripheral embolism, and Alzheimer’s dementia4. The link between PFO and stroke was first described by Cohnheimin 1877 [11], and since then, a strong association has been established. The PFO diameter (average, 4.9 mm) allows the passage of emboli from the venous system that are large enough to conclude up to the middle cerebral artery stem (3 mm) to reach the cerebral circulation [12]. Based on transesophageal echocardiographic (TEE)screening, patients with a cryptogenic stroke/transient ischemic attack (TIA) have an average prevalence of any PFO, PFO associated with atrial septal aneurysm (ASA),and large PFO of 43.2%, 14.5%, and 19.5%, respectively. Importantly, the prevalence of any PFO, PFO with septal aneurysm, and large PFO showed a remarkable variability between younger(<50 years) versus older patients: 59.9%vs. 35.2%, 16.3% vs. 11.6%, and 18.6% vs. 22.9%, respectively; suggesting that PFOs tend to close over time, with larger PFOs tending to persist into older age [13].On the other hand, many PFOs in stroke patients represent incidental findings [14]. Thus, it is essential to determine the high-risk features of PFOs, as only PFO-related CS patients will potentially benefit from a PFO-closure procedure [9,15,16].

According to the recently published guidelines of the German Society of Neurology, all “patients between 16and60 years of age with a cryptogenic ischemic stroke and PFO with moderate or pronounced shunt are recommended a PFO closure” [17]. These national recommendations are also mirrored internationally [18]. On the other hand, all of these guidelines do not cover the extent of neurological and cardiological assessment required prior to PFO closures, especially regarding the diagnostic procedures to rule out AF, i.e., by prolonged electrocardiogram (ECG)screening. Therefore, there is no comment on the need for screening for AF.
Case report: A 35-year-old lady, not known to have diabetes, hypertension, obstructive airway disease or coronary artery disease presented us with complaints of weakness of right upper limb and right lower limb for 7 hours. A non-smoker, who had not taken oral contraceptives for years, the patient did not have any family background of neurological disease. She also denied any headache, vomiting, altered consciousness, speech or swallowing difficulties. She admitted that she had similar types of attach 2 weeks back which resolved of its own within 4 hours. Physical, cardiologic, and neurologic examinations in the Emergency Department were normal, except for the right hemiparesis and mild motor dysphasia. A cranial computed tomography without contrast showed no abnormalities. She was then submitted to a magnetic resonance imaging (MRI) of the brain which also failed to reveal any lesion. Carotid and vertebral Doppler scans were normal. Laboratory tests included normal levels of erythrocyte sedimentation rate, antithrombin III activity, protein C and S antigen, plasma homocysteine, determination of antinuclear factor and anti-DNA antibodies, VDRL and HIV antibodies, extractable nuclear antigen profile, determination of anti-cardiolipin antibodies, lupus anticoagulant, thyroid stimulating hormone, Leiden V factor study, lipids as well as a normal complete blood count and routine blood chemistries. ECG showed sinus rhythm. The contrast-enhanced transesophageal echocardiography showed negative echo contrast at PFO level (diameter <3 mm) (Figure 2 & 3).

Patient refused for CT angiography of cerebral vessels. Within next 16 hours her weakness completely resolved. The patient was subsequently started on vascular protection: clopidogrel 75 mg daily, rosuvastatin 20 mg daily and rabeprazole 20 mg daily. The patient was referred to a tertiary centre for percutaneous closure of PFO.
Discussion
Cryptogenic Stroke (CS) comprises 15-40% of all ischemic strokes, and PFO occurs in 40-56% in patients <55 years old with CS or transient ischemic attack (TIA) [9,19,20]. One has to distinguish between PFO being a direct cause of stroke and PFO being a risk factor for stroke. Depending on the criteria used for diagnosis and the technologyused in cardiac assessment, the prevalence of PFO inthe healthy population is approximately 20- 25% [5,21,22,23,24]. Thus, detection of a PFO during evaluation of a patient with a stroke is not surprising, and thefrequency of PFO detection in these patients can be ashighas40-45% [25]. This frequency of detection is especially high among people without any other obvious explanation for the stroke. Over all, et al. [26] concluded from a meta-analysis of several studies that the relative risk of stroke compared to non-stroke controls increased by a factor of 1.83 if a PFO was present.
The probability that stroke is attributable to PFO is higher in younger than in older patients [27], in the absence of clinical risk factors for stroke than in the presence of such risk factors [28], and in caseof a cortical infarct (suggesting an embolic mechanism) [29]. Notwithstanding, the association between PFO and stroke risk has been documented albeit less strongly in Nolder patients and in patients with classical stroke risk factors, as well as in non-cortical brain infarcts [30,31,32]. The presence of atrial septal aneurysm (ASA) seen in approximately 30% of PFO cases33 also increases the likelihood that PFO is implicated in stroke [27]as well as the risk of recurrent stroke [34].
Additionally, a causal relationship between PFO and stroke is more likely in the presence of larger PFOs (defined as the maximum separation of the septum primum and septum secundum ≥2 mm), longer tunnel length (defined as a maximum overlap of the septum primum and septum secundum ≥8 mm), and/or severe shunting (defined as >30 microbubbles on contrast echocardiography) [35,36]. Another clinical clue suggesting paradoxical embolism in the presence of PFO is concomitant venous thromboembolism (VTE) [37]. Other less-well established factors predisposing to paradoxical embolismin the setting of PFO include straining prestroke, waking up with a TIA or stroke, and obstructive sleep apnea [38]. Accordingly, the risk criteria for stroke in patients with aPFO can be broadly categorized into clinical criteria (at the patient level), PFO anatomical/functional criteria, cerebral imaging criteria, and circumstantial criteria (e.g., concomitant VTE or straining prestroke).
Presumably, most of the neurologic symptoms are secondary to paradoxical embolism of small thrombi that arise in the venous system and pass through the PFO during a transient right-to-left shunt. However, there is normally no direct evidence for paradoxal embolism and systematic screening for deep venous thrombosis in the lower limbs or pelvishasled to extremely variable estimates. Other possibl explanations for stroke secondary to PFO, but independent of paradoxical embolism, include, secondary cardiac arrhythmias or abnormalities of the endocardial surface of the septum or within the PFO which are a focus for thrombus formation [39,40]. In short, the mechanism of strokes among young people with PFO is ill-defined.
Patients with PFO are usually younger and have a lower incidence of hypertension, hypercholesterolemia, and smoking habits than do people with other causes of strokes [21]. Although PFO can be found inolder patients, these people have a high prevalence of atherosclerosis or other cardiac diseases, including atrial fibrillation, which could explain their vascular events. Thus, a PFO might not be perceived asbeing as important in an older person as in a young adult [22,26]. Fukujima, et al. [41], in their transversal trans esophageal echocardiography (TEE) study of 523 patients without any prior evidence of cardiac abnormality, concluded that TEE, widely used to diagnose cardiac source of cerebral embolism in young patients, seems to be useful for patients aged over 45, in whom risk of cerebral embolism is underestimated. Because most people with PFO never have symptoms, some lesions can be assumed to be associated with a greater risk of strokes than others. Establishing a relationship between the size of the sept lab normality, a concomitant atrial septal aneurysm, the presence of a shunt at rest, or the size of the right-to-left shunt might identify those people at greatest risk.
With the use of contrast-enhanced TEE, the PFO is detected frequently during evaluation of patients with an ischemic stroke. The TEE without contrast showed a high sensitivity (90%) and specificity (93%) for detecting PFO whereas contrast transthoracic echocardiography has significant limitations for visualizing the atrial septum [42,43,44]. The use of different contrasts (aerated colloid solution) has increased the sensitivity of echo graphic techniques for diagnosing shunts through the foramen oval. According to Mesa, et al. [42], a greater mobility of the membrane of the oval cavity and a large degree of shunt contrast, as well as shunt at rest detected by saline-contrasted TEE, seems to identify PFO with ischemic stroke. Negrão, et al. [45] considered the transcranial Doppler as another valuable diagnostic tool to detect abnormalities of interatrial septum.
A right-to-left shunt is considered present if microbubbles are detected within 3 to 5 cardiac cycles [46]. The volume or number of bubbles is frequently used to quantify the size of the shunt. However, correlations between the size of the right-to-left shunt and the risk of stroke are not strong. Schuchlenz, et al. 14evaluated PFO size in a series of patients who subsequently underwent catheter closure of the defect and found that the balloon diameter of the PFO was considerably larger than the diameter estimated by TEE. They also reported that a PFO diameter greater than 4 mm was associated with increased thromboembolic risk. De Castro, et al. [47] noted a strong correlation with the risk of embolization when a PFO is associated with a highly mobile septal membrane.
Regardless of the presence of a PFO, the perceived risk of recurrent strokes among patients with symptomatic strokes is so high that some stroke prophylaxis regimen should be prescribed. The choices of antiplatelet aggregating agents, oral anticoagulants, transcatheter placement of an occlusive device or cardiac surgery present a broad range of options which entail different risks and vary considerably in economic costs. Hicken bottom emphasized that “for most patients with PFO, treatment with aspirin for antiplatelet therapy would seem to provide a reasonable approach; for patients with PFO who maybe at a higher risk of paradoxical embolism (underlying hyper coagulable state, recurrent embolic events), alternative options of anticoagulation, combined anti platelet therapy or percutaneous closure could be pursued” (Anticoagulation in acute ischemic stroke - AAN Annual Meeting of San Francisco, 2004).
The PICSS study [48] showed no statistically significant difference in mortality or frequency of recurrent stroke between patients treated with adjusted-dose warfarin at 2 mg or aspirin at 300 mg, both taken once daily. The results of PICSS support those reported by Mas et al. [34]. Despite the expectation that warfarin would be superior to aspirin in preventing recurrent stroke, no data are currently available to support this assumption. Thus, the role of these medications is limited to treating patients with proven venous thrombus or coagulopathy.
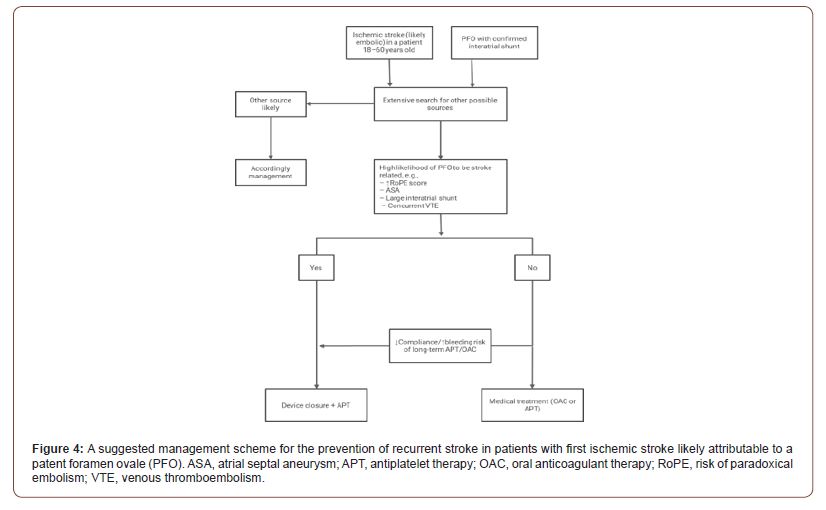
Surgical or endovascular closure could be the best option in very young patients if we consider the risks of a lifelong anticoagulation or antiplatelet therapy [49,50,51]. Mechanical closure of the PFO has been proposed as the definite way to prevent recurrent paradoxical embolism. Some carefully selected patient might benefit from surgery, but currently the features that would necessitate direct operative closure are not known. With the advances in transcatheter endovascular placement of closure devices, the role of major cardiac surgery seems to be diminishing. The procedure has several advantages. It can be done on an outpatient basis using local anesthesia and usually takes less than 30 minutes. The PFO can be closed completely in approximately 95% of patients [21] (Figure 4).
Gilon, et al. [52] concluded, in their case-control study, that mitral valve prolapse is considerably less common than previously reported among young patients with stroke or transient ischemic attack and no more common the naming controls. Using specific and currently accepted echocardiographic criteria, they could not demonstrate an association between the presence of mitral valve prolapse and acute ischemic neurologic events in young people.
In conclusion, interatrial septal abnormalities, including PFO, have been postulated as an additional risk factor for embolism among young ischemic stroke patients. Although its role is a matter of debate and earlier studies have suggested that PFO is an incidental finding, case studies have shown a higher prevalence of PFO in young stroke patients than in the controls. However, large studies need to be conducted to support the connection between interatrial septal abnormalities and ischemic stroke in young adults.
Conclusion
In conclusion, interatrial septal abnormalities, including PFO, may represent an incidental finding, a risk factor or a robust cause in young patients with ischemic stroke or TIA. Although its role is a matter of debate and earlier studies have suggested that PFO is an incidental finding, case studies have shown a higher prevalence of PFO in young stroke or TIA patients than in the controls. PFO is associated with cryptogenic stroke through several mechanisms; most theories support paradoxical embolism, in situ thrombus formation, and arrhythmogenesis, while other possible, yet unknown, explanations cannot be excluded. Young age, PFO morphological characteristics and factors predisposing to venous thrombosis are essential features to determine a pathogenic PFO. However, large studies need to be conducted to support the connection between interatrial septal abnormalities and ischemic stroke and TIA in young adults.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Ferro JM (2003) Cardioembolic stroke: an update. Lancet Neurol 2(3): 177-188.
- Falk RH (1991) PFO or UFO? The role of a patent foramen ovale in cryptogenic stroke. Am J Journal 121: 1264-1266.
- Rana BS, Shapiro LM, McCarthy KP, Ho SY (2010) Three-dimensional imaging of the atrial septum and patent foramen ovale anatomy: defining the morphological phenotypes of patent foramen ovale. Eur J Echocardiogr 11(10): i19–25.
- Aggeli C, Verveniotis A, Andrikopoulou E, Vavuranakis E, Toutouzas K, et al. (2018). Echocardiographic features of pfos and paradoxical embolism: a complicated puzzle. Int J CardiovasImag 34: 1849-1861.
- Hagen PT, Scholz DG, Edwards WD (1984) Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clinic Proc 59: 17-20.
- Naqvi N, McCarthy KP, Ho SY (2018) Anatomy of the atrial septum and interatrial communications . J Thor Dis 10: S2837–S47.
- Menillo AM, Lee L, Pearson-Shaver AL (2020) Atrial Septal Defect (asd). Treasure Island FL: Statpearls.
- Windecker S, Stortecky S, Meier B (2014) Paradoxical embolism. J Am Coll Cardiol 64(4): 403-415.
- Melkumova E, Thaler DE (2017) Cryptogenic stroke and patent foramen ovale riskassessment. Int Card Clin 6(4): 487-493.
- Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, et al. (2013) An indexto identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 81: 619-665.
- Collado FMS, Poulin MF, Murphy JJ, Jneid H, Kavinsky CJ (2018) Patent foramen ovale closure for stroke prevention and other disorders. J Am Heart Assoc 7(12):
- Saver JL, Mattle HP, Thaler D (2018) Patent Foramen Ovale Closure Versus Medical Therapy for Cryptogenic Ischemic Stroke: A Topical Review. Stroke 49(6): 1541-1548.
- Katsanos AH, Bhole R, Frogoudaki A, Giannopoulos S, Goyal N, et al. (2016) The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology 87(10): 988-995.
- Schuchlenz HW, Weihs W, Horner S, Quehenberger F (2000) The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med 109(6): 456-462.
- Maggiore P, Bellinge J, Chieng D, White D, Lan NSR, et al. (2019) Ischaemic stroke and the echocardiographic “bubble study”: are we screening the right patients? Heart, Lung Circul 28(8):1183-1189.
- Thaler DE, Ruthazer R, Weimar C, Mas JL, Serena J, et al. (2014) Recurrent stroke predictors differ in medically treated patients with pathogenic vs. other pfos. Neurology 83(3): 221-226.
- Diener HC, Gerloff C, Thaler DE, Wöhrle J (2018) Closure of Patent Foramen Ovale and Cryptogenic Stroke: unresolved Issues. Curr Neurol Neurosci Rep 18(12): 92.
- Kuijpers T, Spencer FA, Siemieniuk RA, Vandvik PO, Otto CM, et al. (2018) Patent foramen ovaleclosure, antiplatelet therapy or anticoagulation therapy alone for management of cryptogenic stroke? A clinical practice guideline. BMJ 362: k2515.
- Maggiore P, Bellinge J, Chieng D, White D, Lan NSR, et al. (2019) Ischaemic stroke and the echocardiographic “bubble study”: arewe screening the right patients? Heart, Lung Circul 28(8):1183-1189.
- Bayar N, Arslan S, Cagirci G, Erkal Z, Ureyen CM, et al. (2015) Assessment ofmorphology of patent foramen ovale with transesophageal echocardiographyin symptomatic and asymptomatic patients. J Stroke Cerebrovasc Dis 24(6): 1282-1286.
- Meier B, Lock JE (2003) Contemporary management of patent foramen ovale. Circulati on 107: 5-9.
- Meissner I, Whisnant J, Khandheria BK, et al. (1999) Prevalence of potentialrisk factors for stroke assessed by transesophageal echocardiographyand carotid ultrassonography: the SPARC Study. Mayo Clin Proc 74(9): 862-869.
- Lechat P, Mas JL, Lascault G et al. (1988) Prevalence of patent foramen ovalein patients with stroke. N Engl J Med 318(18): 1148-1152.
- Silva MTT, Rodrigues R, Tress J, Victer R, Chamiê F (2005) Patent foramenovale in a cohort of young patients with cryptogenic ischemic stroke.Arq Neuropsiquiatr 63(2B): 427-429.
- Lamy C, Giannesini C, Zuber M, et al. (2002) Clinical and imaging findingsin cryptogenic stroke patients with and without patent foramen ovale:the PFO-ASA Study. Stroke 33(3): 706-711.
- Overell JR, Bone I, Lees KR (2000) Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 55(8): 1172-1179.
- Alsheikh-Ali AA, Thaler DE, Kent DM (2009) Patent foramen ovale in cryptogenic stroke: incidental or pathogenic?Stroke. 40(7): 2349-2355.
- Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, et al. (2013) An index to identifystroke-related vs incidental patent foramenovale in cryptogenic stroke. Neurology 81(7): 619-625.
- Thaler DE, Ruthazer R, Di Angelantonio E, DiTullio MR, Donovan JS, et al. (2013) Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 44(3): 675-680.
- Mazzucco S, Li L, Binney L, Rothwell PM (2018) Oxford Vascular Study PhenotypedCohort.Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack andnon-disabling stroke at older ages: apopulationbased study, systematic review, andmetaanalysis.Lancet Neurol 17(7): 609-617.
- Feurer R, Sadikovic S, Esposito L, Schwarze J, Bockelbrink A, et al. (2009) Lesion patterns in patients with cryptogenic stroke withand without right-to-left-shunt.Eur JNeurol 16(10): 1077-1082.
- Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, et al. (2013) Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 44(12): 3350-3356.
- Dalen JE, Alpert JS (2018) Which Patent ForamenOvales Need Closure to Prevent Cryptogenic Strokes? Am JMed 131(3): 222-225.
- Mas JL, Arquizan C, Lamy C, Zuber M, CabanesL, et al. (2001) Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrialseptal aneurysm, or both. N Engl JMed 345(24): 1740-1746.
- Steiner MM, Di Tullio MR, Rundek T, Gan R,Chen X, et al. (1998) Patent foramen ovale size and embolic brain imaging findingsamong patients with ischemic stroke. Stroke 29(5): 944-948.
- Goel SS, Tuzcu EM, Shishehbor MH, deOliveira EI, Borek PP, Krasuski RA, et al. (2009) Morphology of the patent foramen ovale inasymptomatic versus symptomatic (stroke ortransientischemicattack)patients.AmJCardiol103(1): 124-129.
- Lapergue B, Decroix JP, Evrard S, Wang A,Bendetowicz D, et al. (2015) DiagnosticYield of Venous Thrombosis and Pulmonary Embolism by Combined CTV enography and Pulmonary Angiography in Patients with Cryptogenic Stroke and Patent Foramen Ovale. Eur Neurol 74(1-2): 69-72.
- Ozdemir AO, Tamayo A, Munoz C, Dias B,Spence JD (2008) Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism.J NeurolSci 275(1-2): 121-127.
- Berthet K, Lavergne T, Cohen A, et al. (2000) Significant association of atrialvulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke 31: 398-403.
- Halperin JL, Fuster V (2002) Patent foramen ovale and recurrent stroke: another paradoxical twist. Circulation 105: 2580-2582.
- Fukujima MM, Tatani SB, Aguiar AS, et al. (2005) Transesophageal echocardiography discloses unexpected cardiacsources of embolus instrokepatients agedmore than 45 years. Arq Neuropsiquiatr 63: 941-945.
- Mesa D, Franco M, Lezo JS (2003) Prevalence of patent foramen ovalein young patients with cryptogenic stroke. Rev EspCardiol 56:662668
- Pearson AC, Labovitz AJ, Tatineni S, Gomez C (1991) Superiority oftransesophagealecho cardiography in detecting cardiac source ofembolismin patients with cerebral ischemia of uncertainetiology. Jam CollCardiol 17(1): 66-72.
- Lee RJ, Bartakis T, Yoeh T, Grogin HR, Choi D (1991) Enhanced detectionofintracardiacsources ofcerebral emboli by tranesophagealecho cardiography. Stroke 22: 734-739.
- Negrão EM, Brandi IV, Nunes SV, Beraldo PSS (2005) Alterações do septo interatrial e acidente vascularcerebral isquêArq Neuropsiquiatr 63: 1047-1053.
- Horton SC, Bunch TJ (2004) Patent foramen ovale and stroke. Mayo Clin Proc 79: 35-41.
- S De Castro, D Cartoni, M Fiorelli, M Rasura, A Anzini, et al. (2000) Morphological and functional characteristics of patent foramen ovale and their embolicimplications.Stroke 31(10): 2407-2413.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP (2002) PFO in Cryptogenic Stroke (PICSS) Investigators. Effect of medical treatmentin stroke patients with patent foramen ovale: patent foramen ovale inCryptogenic Stroke Study. Circulation 105:2625-2631.
- Silva MTT, Rodrigues R, Tress J, Victer R, Chamiê F (2005) Patent foramen ovale in a cohort of young patients with cryptogenic ischemic stroke. Arq Neuropsiquiatr 63(2B): 427-429.
- Naess H, Nyland HI, Thomassen L, Aarseth J, Myhr KM (2004) Long-termoutcome of cerebral infarction in young adults. Acta Neurol Scand 110(2): 107-112.
- Homma S, Di Tullio MR, Sacco RL, Sciacca RR, Smith C, et al. (1997) Surgical closure of patent foramen ovale in cryptogenic stroke patients. Stroke 28(12): 2376-2381.
- Gilon D, Buonanno FS, Joffe MM, M Leavitt, J E Marshall, et al. (1999) Lack of evidence of an association between mitral-valve prolapse and stroke in young patients. N Engl J Med 341(1): 8-13.
-
Richmond R Gomes*. Recurrent Transient Ischemic Attack in A Young Lady with Embolic Stroke of Undetermined Source and Patent Foramen Ovale: Quo Vadis- Direct Cause, Risk Factor, or Incidental Finding?. On J Cardio Res & Rep. 7(2): 2023. OJCRR. MS.ID.000660.
-
Cardiac Tumors, Osteosarcoma, Heart, Metastasis, Trans-thoracic echocardiography, Pre-chemotherapy, Oncology, Pulmonary metastasis, Angiography, CTA, Ventriculography, Myocardium
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






